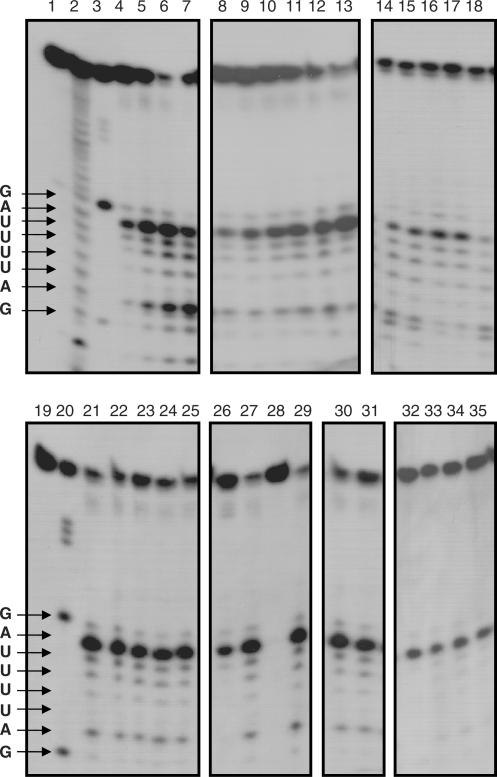
Figure 5.
Base specificity and enzymatic characterization of human RNase κ. A 30-mer 5′-labeled oligoribonucleotide of known sequence was incubated without RNase κ (lanes 1 and 19), in alkaline hydrolysis buffer (lane 2) or with 1 U of ribonuclease T1 (lanes 3 and 20). The same amount of substrate was incubated at 37°C with different amounts of purified recombinant protein (20, 50, 100, 200 ng) for 30 min (lanes 4–7, respectively) or with 30 ng of purified recombinant protein for 10, 20, 30, 40, 50 and 60 min (lanes 8–13, respectively). For the determination of the optimum pH of the human RNase κ, the 5′end-labeled probe was incubated with 25 ng of recombinant protein in 50 mM acetate buffer pH 5.0 and 6.0 (lanes 14 and 15) or 50 mM Tris–HCl pH 7.0, 8.0 and 9.0 (lanes 16–18). For the enzymatic characterization of the human RNase κ, the 5'end labelled probe was incubated with 25 ng of recombinant protein (except indicated otherwise) in 20 mM Tris–HCl pH 8.0 at 37°C for 30 min (lane 21); or in the presence of 5 mM EDTA (lane 22); 100, 150 and 250 mM NaCl (lanes 23–25); 2 mM Cu+2, Mg+2, Zn+2 and Mn+2 (lanes 26–29, respectively); 50 mM NEM or iodoacetamide (lanes 30 and 31, respectively). Lanes 32–35: 10 ng human RNase κ with 0, 10, 20 and 40 U human placental inhibitor (RNasin). The partially digested RNA probe was separated by 8 M urea–17% polyacrylamide gel electrophoresis and visualized by autoradiography.