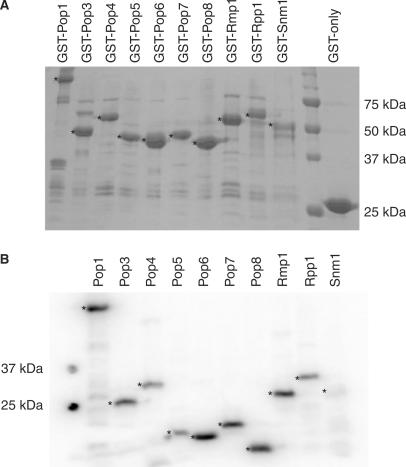
Figure 1.
Preparation of RNase MRP protein subunits. (A) Expressed GST-fusion proteins bound to glutathione Sepharose 4B beads. The expression and purity of GST-fusion protein preparations were determined by SDS-PAGE analysis and Coomassie staining. The asterisks (*) indicate the full-length GST-(fusion) proteins. The bands seen beneath the full-length GST-(fusion) proteins probably represent truncated versions of the full-length recombinant proteins. The sizes of the molecular weight markers are shown on the right. (B) Radiolabelled, cleaved proteins. Whilst bound to glutathione Sepharose, GST fusions were treated with bovine heart kinase in the presence of γ-32P-ATP to achieve radiolabelling, followed by removal of the GST-tag by overnight cleavage with PreScission protease. The efficiency of radiolabelling and the purity of the cleaved proteins were assessed by SDS-PAGE analysis followed by exposure to PhosphoImager screens and analysis using a Typhoon scanner. The asterisks (*) indicate the radiolabelled, untagged proteins. The sizes of the molecular weight markers are shown on the left.