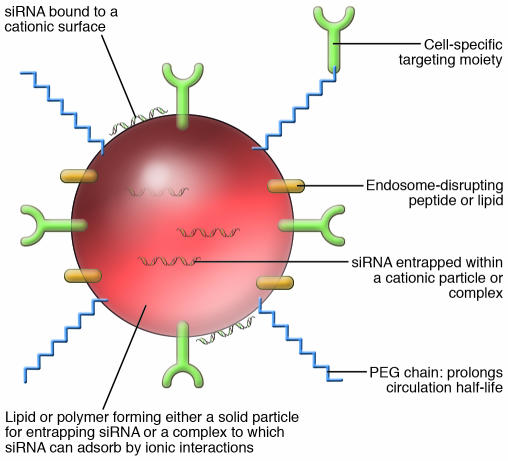
Figure 2. A schematic depiction of the structural components required of a targeted cationic complex or nanoparticle for siRNA delivery in vivo.
Ordered assembly of the complex or nanoparticle (~100 nm in size) typically involves the use of a biocompatible/genocompatible lipid or polymer core that enters tissues and cells readily. The siRNA can be either entrapped within the core, adsorbed onto the surface via ionic interactions, or covalently attached through the sense strand to one of the surface components. The particle can then be modified to bear PEG polymer chains that improve the pharmacokinetic and biodistribution behavior by prolonging half-lives in vivo. PEGylation also serves to improve the biocompatibility of the particles and, because of shielding of the negative charges, to decrease nonspecific interactions with extracellular matrix components. Targeting to a specific tissue or cell type can be facilitated by addition of a receptor ligand or antibody that may be attached to the surface or conjugated to the PEG. Following uptake by endocytosis, intracellular bioavailability can be enhanced through endosomal escape of siRNA by including a fusogenic lipid or pH-sensitive peptide in the complex or nanoparticle (see text for specific examples).