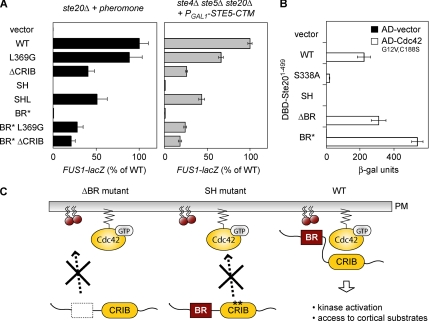
Figure 3.
The BR domain is required for Cdc42-dependent regulation of Ste20. (A) Mutations that disrupt the autoinhibitory conformation of Ste20 suppress the requirement for the BR domain. At left, response to pheromone was tested in ste20Δ cells (PPY913) harboring the indicated GFP-Ste20 alleles (pRS316, pPP538, pPP1117, pPP1010, pPP1009, pPP1109, pPP2204, pPP2239, or pPP2238). Results (mean ± SD; n = 14) were normalized to WT Ste20 (100% = 56 β-galactosidase units). At right, the ability of the same GFP-Ste20 alleles to mediate pheromone- and Gβγ-independent signaling was tested by expression of PGAL1-STE5-CTM (pPP477) in ste4Δ ste5Δ ste20Δ cells (PPY866). Results (mean ± SD; n = 4) were normalized to WT Ste20 (100% = 127 β-galactosidase units). The L369G and ΔCRIB mutations disrupt Ste20 autoinhibition (Lamson et al., 2002); as controls, the SH (S338A H345G) and SHL (S338A H345G L369G) mutations were also tested to compare the ability of L369G to suppress either CRIB or BR domain mutations. (B) BR domain mutations do not affect Cdc42 binding. An activation domain fusion to Cdc42G12V C188S (pPP1027) or vector (pPP244) was coexpressed with DNA-binding domain fusions to the Ste20 N-terminus (DBD-Ste201-499; from top to bottom, pPP167, pPP1053, pPP1059, pPP1061, pPP2950, and pPP2949) in the two-hybrid reporter strain PPY760. Interaction was measured by quantitative β-galactosidase assay (mean ± SD; n = 4). As controls, mutations in the Ste20 CRIB domain that disrupt Cdc42 binding either moderately (S338A) or severely (SH = S338A H345G; Lamson et al., 2002) were assayed in parallel. (C) Schematic model for the Cdc42-dependent regulation of Ste20 at the plasma membrane. In mutants lacking a functional BR domain (ΔBR) or a functional CRIB domain (SH), membrane-localized activation of Ste20 is impaired. Only when both domains are intact (WT) is Ste20 efficiently localized to the plasma membrane, allowing kinase activation and efficient access to cortical substrates.