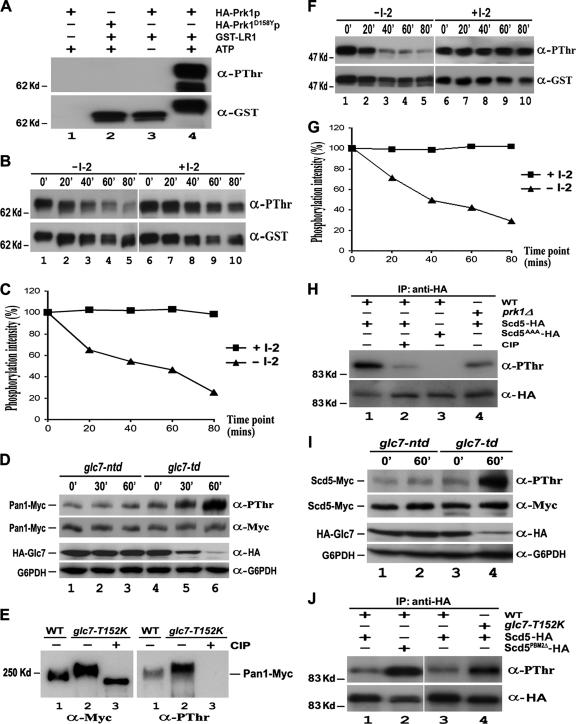
Figure 3.
Dephosphorylation of Pan1p and Scd5p by Glc7p in vitro and in vivo. (A) In vitro phosphorylation of Pan1p by Prk1p. Purified GST-LR1 was incubated with immunoprecipitated HA-Prk1p (lanes 3 and 4) or HA-Prk1D158Yp (lane 2) in the presence (lanes 2 and 4) or absence (lane 3) of nonradioactive ATP at 25°C for 3 h. The mixture was separated by gel electrophoresis and immunoblotted with anti-PThr and then anti-GST antibodies. (B and F) In vitro dephosphorylation of Pan1p (B) and Scd5p (F) by Glc7p. Immunoprecipitated Glc7-HA with or without the I-2 treatment was incubated with in vitro phosphorylated GST-LR1 or GST-SCD5 at 37°C. Samples were taken at 20-min intervals, electrophoresed, and immunoblotted with anti-PThr and anti-GST antibodies. (C and G) Measurement of Glc7p phosphatase activity on Pan1p (C) and Scd5p (G). The phosphorylation level of GST-LR1 (Figure 3B) and GST-SCD5 (Figure 3F) at each time point was measured by densitometer and normalized against its protein amount. (D) Pan1p phosphorylation level in glc7-td mutant. YMC484 (lanes 4–6) and YMC485 (lanes 1–3) cells were allowed to grow to the log phase at 25°C and then shifted to 37°C. Samples were taken at 0, 30, and 60 min, and Pan1p-Myc was immunoprecipitated, electrophoresed, and immunoblotted with anti-PThr and then anti-Myc antibodies. Total proteins of each sample also were extracted by TCA precipitation and subjected to Western analysis to assay the expression level of Glc7p. (E) Pan1p phosphorylation level in glc7-T152K mutant. Pan1-Myc was immunoprecipitated from YMC441 (lane 1) and YMC482 (lanes 2–3) cells grown at 30°C. The immunoprecipitates in lane 3 were preincubated with CIP for 30 min before loading. (H) Phosphorylation of Scd5p in vivo. YMC448 (lanes 1–2), YMC449 (lane 3), and YMC488 (lane 4) cells expressing either Scd5-HA (lanes 1 and 2 and 4) or Scd5AAA-HA (lane 3) were grown at 30°C and incubated with phosphatase inhibitor cocktail for 2 h. The HA-tagged proteins were immunoprecipitated, electrophoresed, immunoblotted with anti-PThr and then anti-HA antibodies. (I) Scd5p phosphorylation level in glc7-td mutant. Log phase YMC492 (lanes 1 and 2) and YMC493 (lanes 3 and 4) cells at 25°C were incubated with phosphatase inhibitor cocktail for 2 h and shifted to 37°C. Samples were taken at 0 and 60 min, and Scd5-Myc was immunoprecipitated, electrophoresed, and immunoblotted with anti-PThr and then anti-Myc antibodies. The expression level of Glc7p in each time point was also assayed by Western analysis. (J) Scd5p phosphorylation level in cells containing either T152K mutation on Glc7p or PBM2Δ mutation on Scd5p. Log phase YMC487 (lanes 1 and 3), YMC490 (lane 4), and YMC489 (lane 2) cells at 25°C were shifted to 37°C for 4 h with phosphatase inhibitor cocktail present during the last 2 h. The HA-tagged proteins were immunoprecipitated, electrophoresed, and immunoblotted with anti-PThr and then anti-HA antibodies.