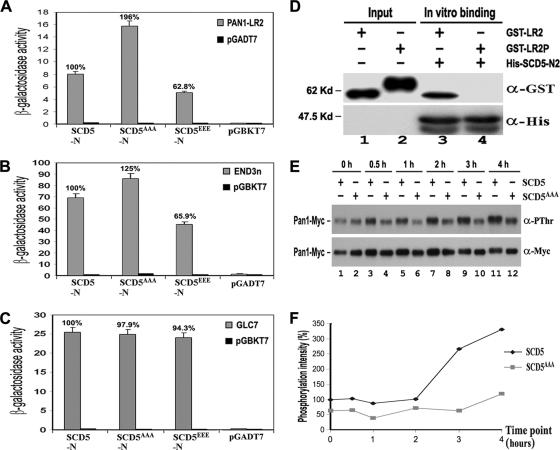
Figure 5.
Phosphoregulation of Scd5p-related interactions. (A–C) Two-hybrid interactions of wild-type (SCD5), SCD5AAA, and SCD5EEE with Pan1p (A), End3p (B), and Glc7p (C). (D) In vitro binding assays between Scd5p and phosphorylated Pan1p. His-tagged SCD5-N2 was immobilized and incubated with unphosphorylated GST-LR2 (lane 3) and phosphorylated GST-LR2P (lane 4), respectively. The precipitates, together with portions of GST-LR2 and GST-LR2P (lanes 1–2), were separated by gel electrophoresis and sequentially immunoblotted with anti-GST and anti-His antibodies. (E) The effect of SCD5AAA mutation on Prk1p-induced Pan1p phosphorylation. YMC471 and YMC473 cells containing pGAL-PRK1-313 were allowed to grow at 30°C to log phase in raffinose, followed by addition of galactose. Samples were taken at the indicated time points. Pan1-Myc was immunoprecipitated, electrophoresed, and immunoblotted with anti-PThr and anti-Myc antibodies. (F) Calculation of the relative phosphorylation intensity of Pan1-Myc in Figure 5E. The phosphorylation level of Pan1-Myc in each lane was measured by densitometer and normalized against its protein amount. The relative phosphorylation intensities of all lanes were calculated against lane 1.