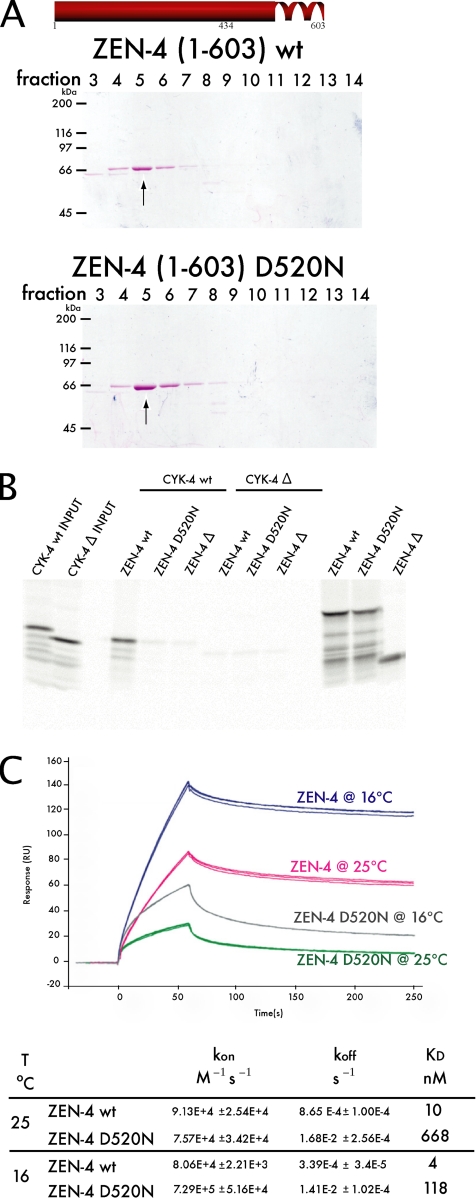
Figure 2.
The primary defect of zen-4(or153ts) is due to the failure of ZEN-4 D520N to form a stable complex with CYK-4. (A) The ability of ZEN-4 D520N to form a dimer was tested by size exclusion chromatography. ZEN-4(1-603)D520N eluted in the same fraction as wt ZEN-4(1-603), thus ZEN-4 D520N is a dimer. (B) CYK-4(1-120) and ΔCYK-4(34-120) fragments were expressed as 35S-labeled proteins by in vitro translation. Also shown (far right lanes) are parallel reactions of ZEN-4 translation reactions with [35S]methionine, as controls for the efficiency of translation. Unlabeled ZEN-4(434-775) wt and D520N fragments, and the negative control ΔZEN-4(475-775), were added and incubated for 1 h. ZEN-4 was subsequently immunoprecipitated with an anti-ZEN-4 antibody. Whereas wt ZEN-4 can bind wt CYK-4, ZEN-4 D520N cannot. Therefore, ZEN-4 D520N is defective in CYK-4 binding, yet is a dimer (A). (C) The affinity and the rates of interaction between ZEN-4 and CYK-4 were measured through SPR. Sensorgrams of triplicate runs representing 100 nM ZEN-4 injected are shown. The affinity of ZEN-4 D520N for CYK-4 is reduced by 60-fold from wt ZEN-4/CYK-4 affinity at 25°C. However, at 16°C the mutant's affinity for CYK-4 improves.