Fig. 1.
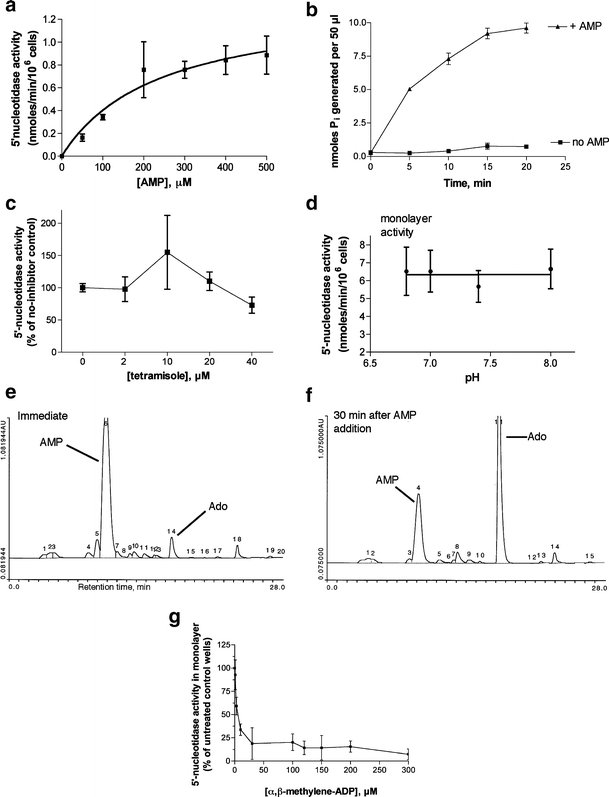
Characterization of ecto-5′-nucleotidase activity in T84 cell monolayers. 5′-Nucleotidase activity was measured by the release of inorganic phosphate (Pi) from AMP in phosphate-free buffer using a colorimetric method as described in “Materials and methods.” For a, c, and d, the spectrophotometric reading in the absence of AMP (“no AMP blank”) was subtracted from the total reading. In b the “no AMP blank” was not subtracted and is shown in b as labeled. a Michaelis-Menten curve of enzyme velocity vs concentration of AMP substrate. b Time course of Pi release vs time. c Lack of inhibition by tetramisole, an inhibitor of alkaline phosphatase. d pH dependence of 5′-nucleotidase activity. e, f HPLC traces showing conversion of AMP (e) to adenosine (Ado, f) in T84 cell monolayers. g Sensitivity of the AMP-hydrolyzing activity to the inhibitor α,β-methylene-ADP, confirming it as ecto-5′-nucleotidase
