Fig. 9.
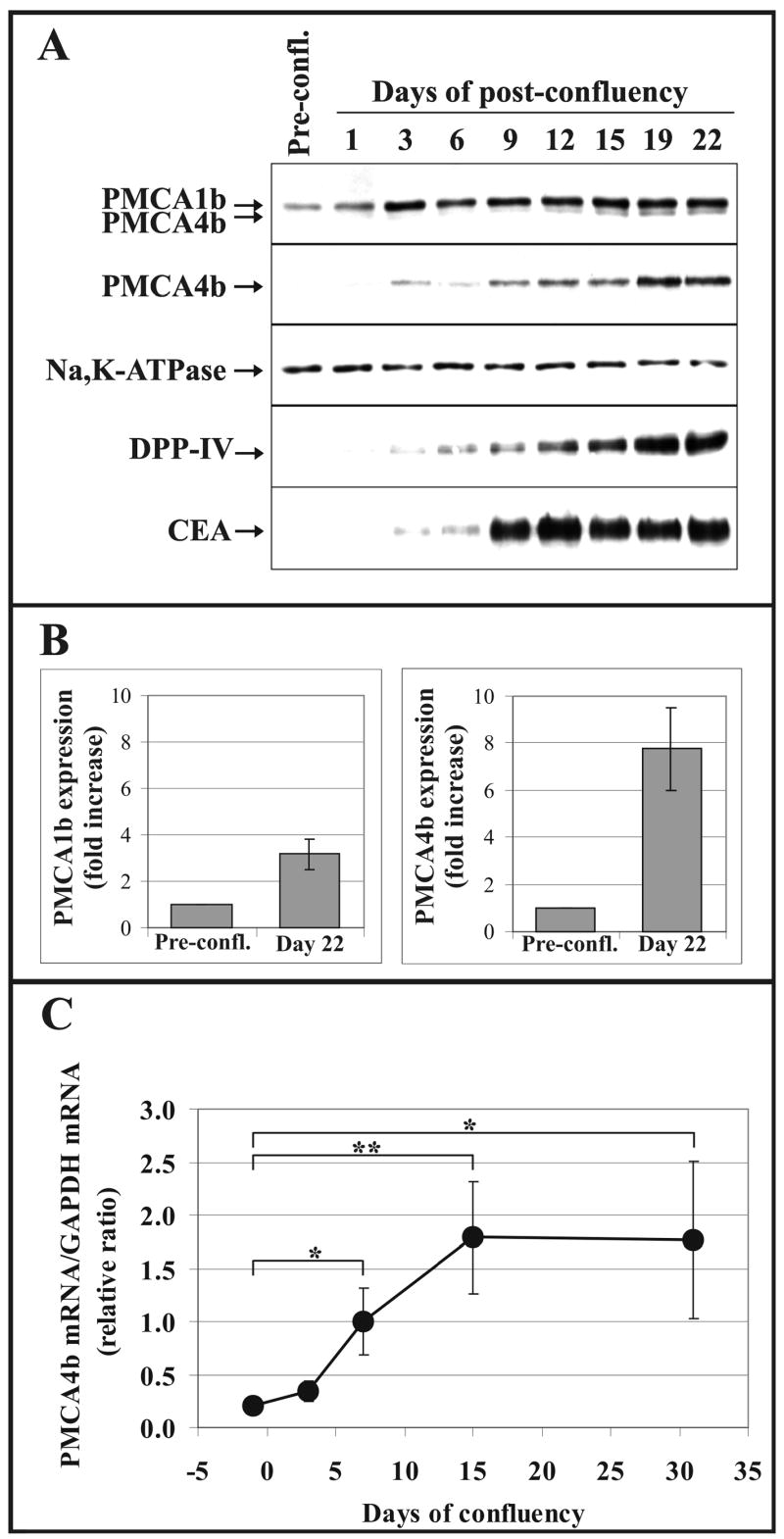
Modulated PMCA expression in differentiating Caco-2 colon cancer cells. Caco-2 cells were allowed to reach confluency and cultured in post-confluency for more than 3 weeks. Cells were harvested at different time points and lysed for both Western-blot analysis (A and B) and real-time PCR analysis (C).
A, Western-blot analysis of the expression of PMCA isoforms by using the pan-anti-PMCA (5F10) and the PMCA4b-specific (JA3) monoclonal antibodies. The expression of Na+/K+-ATPase and two established markers of enterocytic differentiation (DPP-IV and CEA) were monitored in parallel. Equal amounts of total cellular protein harvested at the indicated time points were loaded onto SDS-polyacrylamide gels (from 20 μg to 50 μg, depending on the antibody used for immunostaining).
B, The bars represent the fold increases of the PMCA1b and PMCA4b protein expressions in differentiated Caco-2 cells at day 22 of post-confluency (means ± S.D. of n ≥15 from at least two independent experiments). The expression levels of PMCA1b and 4b in pre-confluent cultures of Caco-2 cells were used as control values for quantifications.
C, Real-time PCR analysis was conducted with PMCA4b-specific primers using: equal amounts of total cDNA templates obtained following reverse transcription of mRNAs from pre-confluent Caco-2 cells or from cells cultured in post-confluency for the time points indicated. As internal control, GAPDH was simultaneously amplified from the same cDNA templates. Normalized PMCA4b/GAPDH mRNA ratios were calculated by using crossing point values and separate calibration curves for the two amplicons, and plotted as a function of time elapsed from confluency. Day 0 is when confluency is reached.
The data in this panel represent the means ± S.D. of at least three separate determinations of a representative experiment. Statistical significance is denoted by when *p<0.05 or **p<0.01. Strong induction of PMCA4b expression was observed both at the protein and mRNA levels during culturing Caco-2 cells in post-confluent conditions that allows differentiation of cells toward an enterocytic phenotype.
