Figure 2.
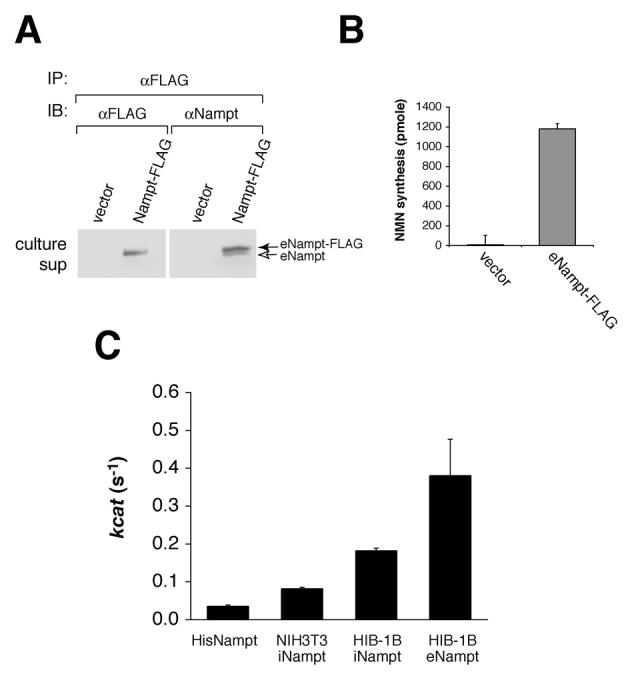
eNampt produced by differentiated HIB-1B brown adipocytes has high Nampt enzymatic activity. A) eNampt-FLAG co-immunoprecipitates with untagged eNampt from culture supernatants. The eNampt-FLAG protein was immunoprecipitated with an anti-FLAG antibody from 8 ml of each culture supernatant and blotted with either the same antibody or an anti-Nampt antibody. The culture supernatants of the vector-transfected cells were used as a control. B) The eNampt-FLAG protein immunoprecipitated from culture supernatants of differentiated Nampt-FLAG HIB-1B cells has Nampt enzymatic activity. Results are presented as mean ± SE (n=3). C) The kcat values of the bacterially produced His-tagged recombinant Nampt and intra- and extracellular Nampt-FLAG from NIH3T3 and differentiated HIB-1B cells were calculated by measuring NMN synthesis and quantifying the amount of Nampt by Western blotting (data not shown). Results are presented as mean ± SEM (n=7 for His-tagged Nampt, 3 for iNampt from NIH3T3, 6 for iNampt from HIB-1B, and 4 for eNampt from HIB-1B), and all differences in pair-wise comparisons are statistically significant with the Student's t test (p < 0.05).
