Figure 3.
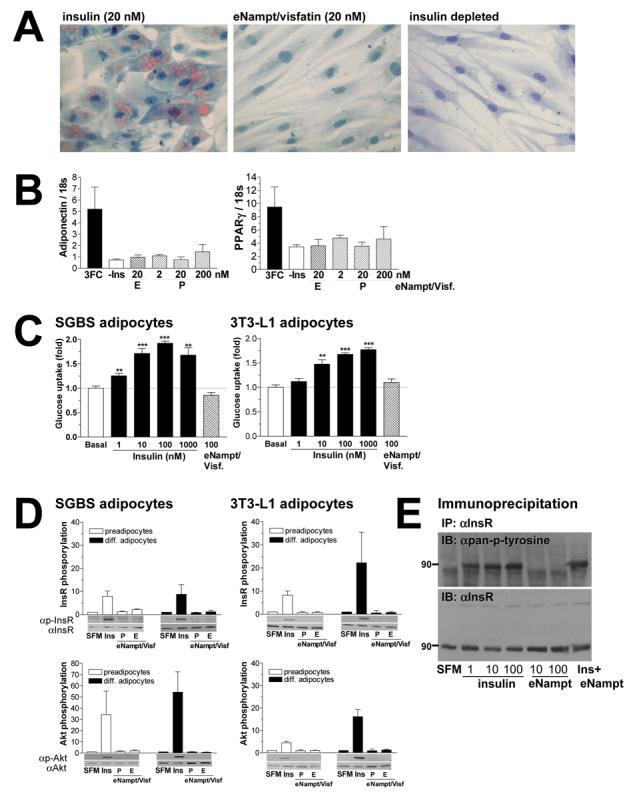
eNampt does not exert insulin-mimetic effects on adipogenesis, glucose uptake, and insulin signaling in cultured cells. A) Differentiation of human SGBS preadipocytes was induced over a period of 8 days in standard induction medium containing 20 nM insulin or 20 nM eNampt/visfatin. For negative control, cells were treated in induction medium without insulin. Mature adipocytes were stained with Sudan III to visualize lipid accumulation at day 8. B) mRNA expression levels of two adipocyte differentiation markers, adiponectin and PPARγ, were analyzed with real-time quantitative RT-PCR. SGBS preadipocytes were differentiated in standard induction medium with 20nM insulin (3FC) or eNampt/visfatin proteins produced in bacteria (P) or in mammalian cells (E) at concentrations indicated. C) Differentiated SGBS and 3T3-L1 adipocytes were incubated with increasing concentrations of insulin or 100 nM eNampt/visfatin produced in mammalian cells. Glucose uptake was determined using [14C]-2-deoxyglucose at 0.5 μCi/ml for 5 min. Experiments were performed in quadriplicates, and glucose uptake was normalized to the amount of protein. Basal glucose uptake in non-stimulated cells is assigned as 1. D) The effect of eNampt/visfatin on phosphorylation of the insulin receptor (InsR, upper panels) and Akt/PKB kinase (Akt, lower panels) was examined in undifferentiated and differentiated human SGBS and mouse 3T3-L1 cells. Cells were starved overnight and then exposed to serum free medium (SFM), 10 nM insulin (Ins), or 10 nM eNampt/visfatin produced in bacteria (P) or in mammalian cells (E). Signals of phosphorylated proteins are normalized to those of non-phosphorylated proteins, and values are shown relative to the signal in serum free medium (n ≥ 3). E) Insulin receptor phosphorylation was examined in R-IR cells treated with different concentrations of insulin and eNampt/visfatin. Cells were treated with the indicated concentrations of insulin, eNampt/visfatin produced in mammalian cells or both (10 nM each). The insulin receptor protein (InsR) was immunoprecipitated with an anti-InsR antibody 29B4 and blotted with an anti-pan-phosphotyrosine antibody (upper panel). The membrane was re-probed with another anti-InsR antibody C19 (lower panel).
All results are expressed as mean ± SEM.
