Figure 6.
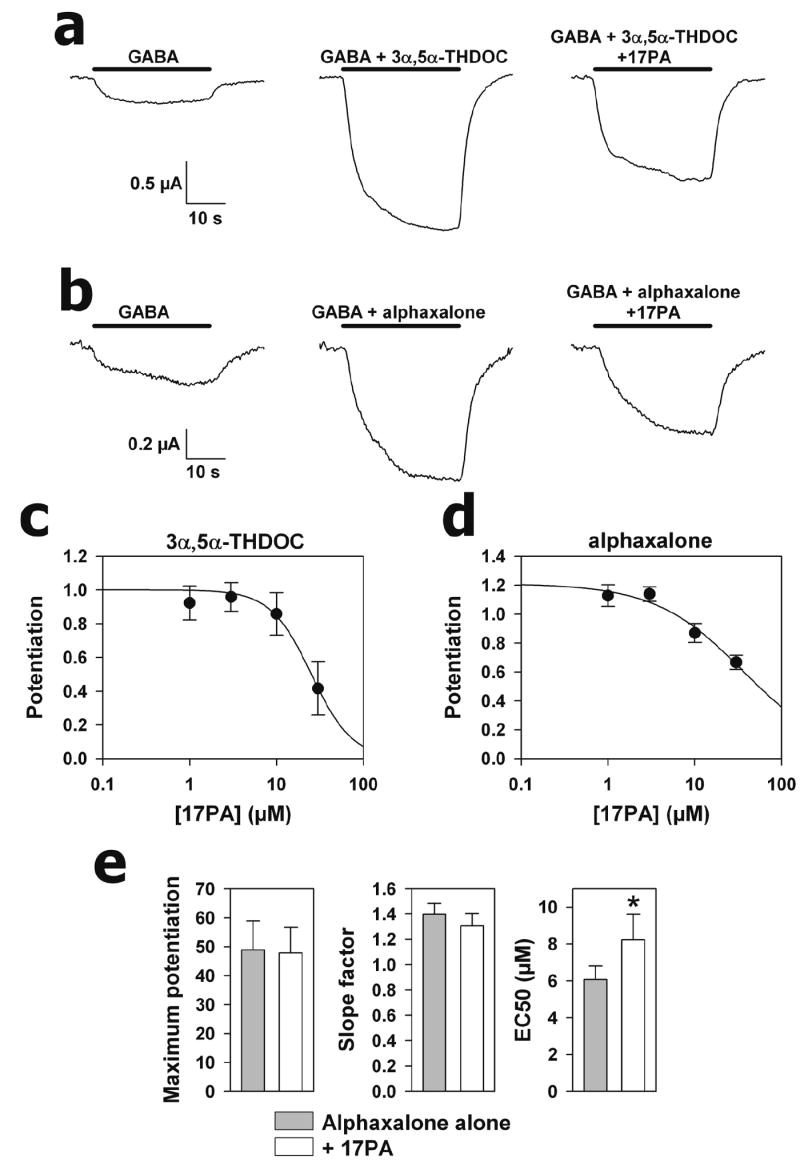
Responses of Xenopus oocytes expressing recombinant rat α1β2γ2 subunits to GABA co-applied with potentiating steroids and 17PA. a. Effect of 17PA on 3α,5α-THDOC potentiation. b. Effect of 17PA on alphaxalone potentiation. Horizontal bars indicate the duration of drug exposure. Oocytes were clamped at -70 mV and drug concentrations were GABA (2 μM), 3α,5α-THDOC (0.5 μM), alphaxalone (0.5 μM) and 17PA (10 μM). Responses in the two panels were obtained from two separate oocytes. c. Inhibition curve for 17PA against 3α,5α-THDOC in oocytes expressing recombinant receptors. 3α,5α-THDOC concentration was constant at 0.5 μM. Potentiation refers to the fractional increase over the baseline GABA response (1.0 denotes a doubling of the response). Error bars denote standard error of the mean. The solid line is a fit predicting an IC50 of 25 μM. d. Similar inhibition curve for alphaxalone (0.5 μM) predicts an IC50 of 36 μM. e. Effects of 17PA on alphaxalone concentration response parameters. The graphs are taken from fitting a concentration response function to 8 oocytes challenged with 0.3 – 10 μM alphaxalone in the absence and presence of 10 μM 17PA. The only significant change was a shift to the right in the alphaxalone EC50, consistent with a competitive or pseudocompetitive mechanism of 17PA.
