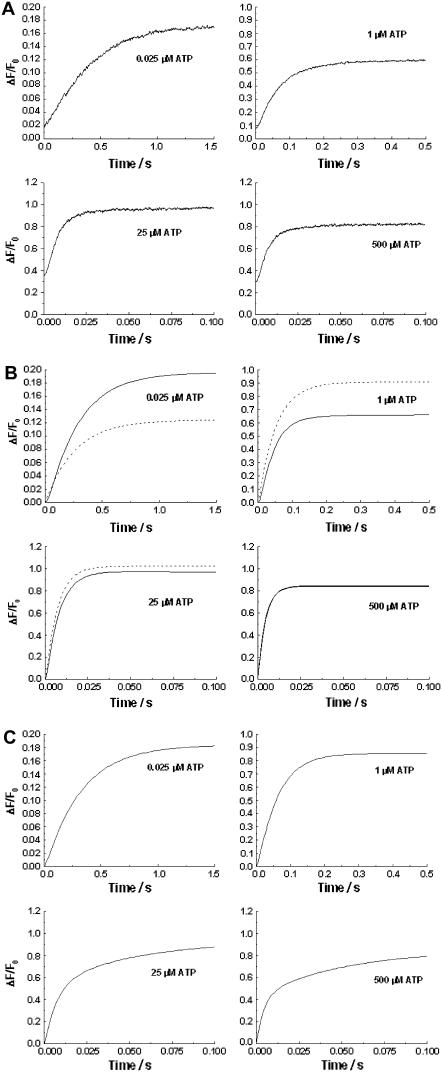
FIGURE 1.
(A) Stopped-flow fluorescence transients of Na+,K+-ATPase from pig kidney noncovalently labeled with RH421 (75 nM, after mixing). Na+,K+-ATPase (10 μg/ml or 68 nM, after mixing) was rapidly mixed with an equal volume of a solution containing varying concentrations of Na2ATP. Both the enzyme suspension and the Na2ATP solutions were prepared in a buffer containing 130 mM NaCl, 30 mM imidazole, 5 mM MgCl2, and 1 mM EDTA (pH 7.4, 24°C). The fluorescence of membrane-bound RH421 was measured at an excitation wavelength of 577 nm at emission wavelengths of ≥665 nm (RG665 glass cutoff filter). The calculated reciprocal relaxation times were: 0.025 μM ATP, 2.57 (±0.03) s−1; 1 μM ATP, 6.51 (±0.04) s−1; 25 μM ATP, 138 (±2) s−1 (96% of the total amplitude) and 12 (±3) s−1 (4%); and 500 μM ATP, 179 (±6) s−1 (89%), and 41 (±6) s−1 (11%). (B) Kinetic simulations of the experimental fluorescence transients based on a single-site monomer model (dashed line, see Fig. 3 a) and a dimer model (solid line, see Fig. 4). The values of the rate constants, equilibrium constants, and fluorescence levels for the dimer model are given in Table 1. (C) Kinetic simulations of the experimental transients based on a two-pool enzyme model (see Fig. 3 b).