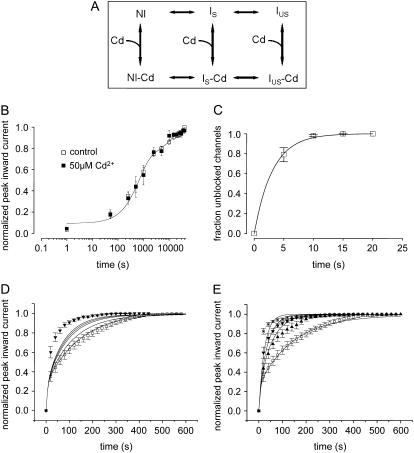
FIGURE 8.
(A) Kinetic modeling of the modulation of IUS by Cd2+. Hypothetical scheme of the proposed kinetic states and transitions. Upper row depicts Cd2+-free states: NI = noninactivated, IS = slow inactivated, IUS = ultraslow inactivated. Lower row: Cd2+-bound states. (B) Time course of recovery from slow inactivation. From a holding potential of −120 mV, channels were depolarized to −20 mV for 25 s. Thereafter, the membrane potential was returned to −120 mV for the indicated time periods after which channel availability was tested by a 20-ms pulse to −20 mV. Peak inward currents were normalized to the value after full recovery. The time course of recovery was determined during a control period and during superfusion with 50 μM Cd2+. Data points were fitted with double exponential functions (Eq. 5). The parameters were drug free: A1 = 0.62 ± 0.04, τ1 = 573.2 ± 32.3 ms, A2 = 0.30 ± 0.02, τ2 = 6493.4 ± 56.3 ms; Cd2+: A1 = 0.61 ± 0.07, τ1 = 663 ± 64.3 ms, A2 = 0.29 ± 0.03 (P = n.s.), τ2 = 6828.5 ± 90.4 ms, n = 3; P = n.s. (C) Time course of relief from block by 50 μM Cd2+ in K1237C channels. K1237C channels were heterologously expressed in tsA201 cells and examined by the whole-cell patch-clamp technique. Cells were held at −120 mV, and inward currents were elicited by 10 ms depolarizations to −20 mV at 20-s intervals. Data points were fitted with a monoexponential function (Eq. 6). See text for fitting parameters. (D) Model A: Modulation of IUS by low-affinity binding of Cd2+ to the IUS state. Data points are time courses of recovery during control and during exposure to 20 μM Cd2+ (i.e., a concentration close to the KD), taken from Fig. 2 C. Lines are model calculations assuming rates of unbinding of Cd2+ from the IUS state to be reduced with respect to the unbinding rates from the IS and NI state by factors 0, 2, 5, 10, 100, and 1000 (lines in order from right to left). The lines representing model calculations assuming unbinding rates 100-fold and 1000-fold lower for IUS than for IS and NI are superimposed. (E) Model B: Modulation of IUS by high-affinity binding of Cd2+ to the IS state. The affinity of Cd2+ to the IS state was assumed to be 10 times higher than the affinities for the NI and the IUS states. Data points are the same as in Fig. 2 D. Lines from right to left represent calculations assuming the following concentrations of Cd2+: 0 μM, 10 μM, 20 μM, and 50 μM.