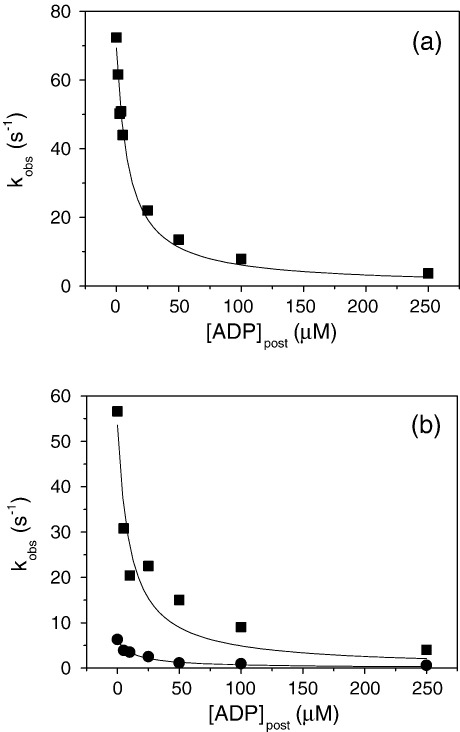
Figure 6.
ATP-induced dissociation of pyr-acto–BMS1 in the presence of ADP. 0.25 μM phalloidin-stabilized pyrene-labelled actin was incubated with an equimolar amount of BMS1 before mixing with variable concentrations of ADP and 50 μM ATP (a) or 200 μM ATP (b). The data could be fitted against a single exponential (a) or a double exponential (b) and the resulting kobs were fitted toa hyperbola, resulting in an apparent affinity (KAD) forADP (a) KAD = 9.6(±1.4) μM (20 °C) and (b) KAD = 6.8(±1.7) μM (▪, fast phase) and KAD = 6.6(±1.9) μM (•, slow phase).