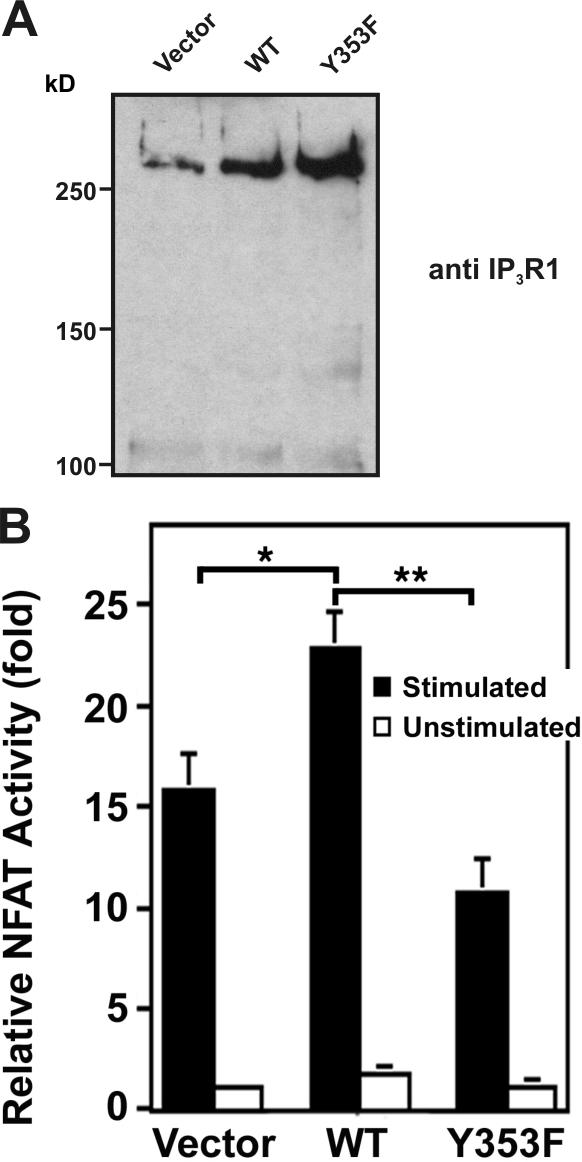
Figure 7.
Effect of IP3R1 Y353 phosphorylation on NFAT activity. (A) Immunoblotting of lysates from 106 transfected Jurkat T cells with anti-IP3R1 antibody showed comparable overexpression of WT-IP3R1 and IP3R1-Y353F. (B) Relative NFAT-dependent luciferase activity. 106 Jurkat cells were transiently transfected with WT-IP3R1, IP3R1-Y353F, or pcDNA3.1 vector together with an NFAT–firefly luciferase reporter assay construct and a TK-Renilla luciferase construct as a control for luciferase transfection efficiency. After stimulation with OKT3 (anti-CD3 antibody), cells were lysed and the lysates were assayed for NFAT-luciferase activity, quenched, and assayed for TK-luciferase activity in triplicate. NFAT activity is expressed as relative NFAT luminescence normalized to TK luminescence and adjusted for IP3R1 expression levels. The results are shown as fold stimulation over nonstimulated vector control. Data were presented as the mean ± SEM of seven independent Jurkat preparations assayed in triplicate. (*, P < 0.05 WT vs. vector by t test; **, P < 0.05 WT vs. Y353F by t test).