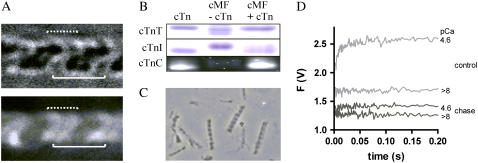
FIGURE 1.
Incorporation of NBD-cTn into guinea pig cardiac myofibrils. (A) Microscopic images (90× magnification) in bright-field (upper) and epifluorescence mode (lower) of a stretched myofibril bundle (consisting of two single myofibrils) exchanged with NBD-cTn. The solid lines indicate the position of a sarcomere limited by the two Z-lines and the dotted lines the position of the thin filaments on both sides of a Z-line. The width of solid lines corresponds to the sarcomere length (3.15 μm). (B) SDS-PAGE of guinea pig cardiac myofibrils (cMF) before and after the exchange with human NBD-cTn (for details, see Materials and Methods). Because endogenous guinea pig cTnC and exchanged NBD-cTnC have the same electrophoretic mobility the incorporation was proved by excitation of NBD-cTn with ultraviolet light. To obtain high enough fluorescence, a 12-fold higher myofibril- and NBD-cTn concentration was used than for cTnI and cTnT. (C) NBD-cTn exchanged myofibrils after being mixed in the stopped-flow apparatus. (D) Myofibrils exchanged with NBD-cTn were incubated either for 1 h with 10 mg/ml unlabeled cTn (“chase”) or in the same buffer but without exogenous cTn (“control”). Thereafter, myofibrils were washed in relaxation buffer (for further details, see Materials and Methods) and mixed with either relaxation buffer (pCa >8) or activation buffer (pCa 4.6). Though the chased myofibrils still exhibit a noticeable high basal fluorescence at pCa >8, the Ca2+-induced fluorescence increase is almost completely lost.