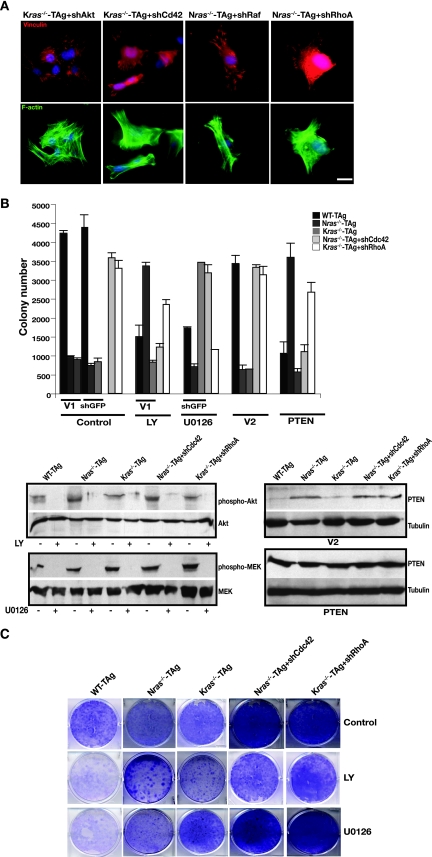
FIG. 6.
Preferential contributions of downstream signaling effectors in ras isoform-deficient MEFs to the actin cytoskeleton, focal adhesions, and transformation. (A) Transformation of ras isoform-deficient cells correlates with specific actin remodeling. Shown is FITC-phalloidin staining (green) for actin, vinculin staining (red) for focal adhesions, and DAPI staining (blue) for nuclei in Kras-deficient MEFs following knockdown of Akt or Cdc42 (denoted shAkt and shCdc42, respectively) and in Nras-deficient cells depleted of Raf or RhoA (indicated by shRaf and shRhoA). Bar, 10 μm. (B) Effects of pharmacological inhibition of PI3K and Raf pathways and PTEN overexpression on the anchorage-independent growth of Nras-deficient Cdc42-depleted MEFs and Kras-deficient RhoA-depleted MEFs. Cells (genotype and expression of shRNA are indicated; V1 is vector) were subjected to a soft agar assay and subsequently treated with the PI3K inhibitor LY294002 (LY; 20 μM) and the MEK inhibitor U0126 (10 μM), as described in Materials and Methods. For PTEN overexpression, cells were infected with virus directing the expression of PTEN or vector (V2; pBabe-GFP), sorted, and subjected to a soft agar assay. Results are means ± standard deviations of two independent experiments. Total cell lysates were subjected to immunoblotting to verify the effectiveness of the pharmacological inhibitors (bottom left panel) and PTEN overexpression (bottom right panel). (C) Same analysis as in panel B, except long-term clonogenicity assays were performed as described in Materials and Methods.