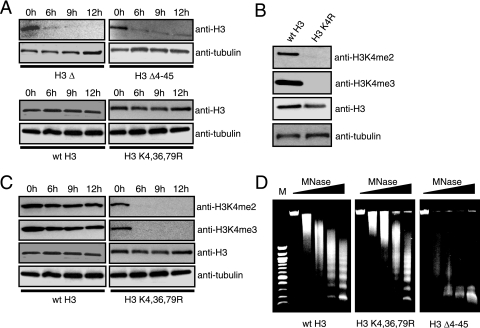
FIG. 2.
The histone H3 K4,36,79R mutant protein is stable and competent for nucleosome assembly. (A) The protein stability of different histone mutants was measured by Western blotting analysis. Cell extracts from each of the mutants at four time points following the depletion of wild-type histone H3 were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed with anti-H3 antibody. As an internal loading control, anti-α-tubulin antibody was used. (B) Antibodies against di- and trimethylated histone H3K4 do not detect the histone H3 K4R mutant while antibody against histone H3 detects both wild-type histone H3 and the H3 K4R mutant. Cell extracts from wild-type and histone H3 K4R mutant strains were analyzed with antibodies as indicated. The level of the α-tubulin subunit was used as an internal loading control. (C) Loss of histone H3K4 methylation at different time points following the depletion of wild-type histone H3. Cell extracts from wild-type histone H3 and the H3 K4,36,79R mutant at different time points following the depletion of wild-type histone H3 were examined by Western blotting analysis with appropriate antibodies, as described in the panel B legend. (D) Genomic chromatin structure of the different histone mutants and wild-type strains was examined by MNase digestion assays. MNase digestion profiles for cells harvested at the 6-h time point following the depletion of wild-type histone H3 are shown as representative patterns. Subsequent lanes had doubling concentration of MNase from 0 to 37.5 units/ml. M, 100-bp DNA molecular size ladder.