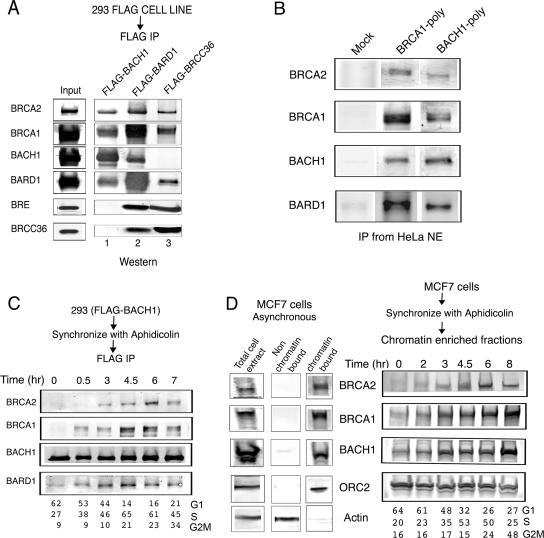
FIG. 1.
Purification of the BACH1-BRCA1 complex. (A) FLAG-BACH1, FLAG-BARD1, and FLAG-BRCC36 were immunoprecipitated from nuclear extracts of the corresponding cells with anti-FLAG-M2 agarose beads. The purified proteins were resolved on 4-12% Tris-glycine gels followed by Western blot analysis using the antibodies shown to the right of the panel. (B) Endogenous BACH1-BRCA1 complex was immunoprecipitated from HeLa nuclear extracts with polyclonal antibodies against BRCA1 and BACH1. The purified proteins were immunoblotted with antibodies against the proteins shown to the right of the panel. (C) Association of BACH1 and BRCA1 during cell cycle progression: the BACH1-BRCA1 interaction is cell cycle dependent. FLAG-BACH1 cells were synchronized with aphidicolin as described in Materials and Methods. The cells were released at the indicated times, followed by immunoprecipitation of nuclear extracts with anti-FLAG antibody. Immunoblot assays were carried out with the antibodies mentioned. Distribution of cells in the cell cycle as examined by flow cytometry is included for each time point. (D) Chromatin association of BRCA2, BRCA1, and BACH1 is cell cycle regulated. MCF7 cells were synchronized with aphidicolin and, at different time points after release, subjected to biochemical fractionation to isolate chromatin-bound proteins as described in Materials and Methods. Immunoblot assays of the chromatin-enriched fractions were carried out with the antibodies indicated. The cell cycle distribution at each time point is shown. Western blot analysis using the total lysate or the chromatin-bound and non-chromatin-bound amounts of BRCA2, BRCA1, BACH1, ORC2, and actin is shown on the right of the panel.