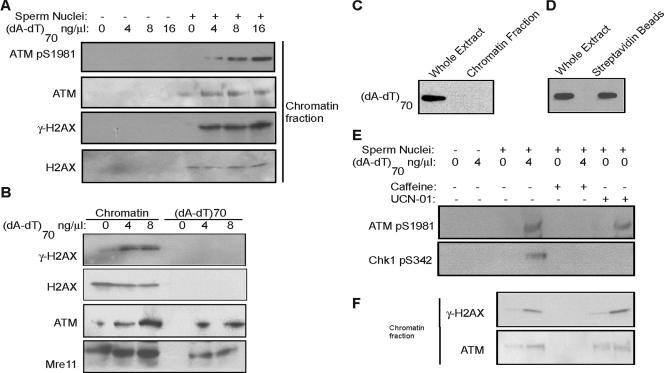
FIG. 3.
DNA damage induces H2AX phosphorylation (γ-H2AX) and ATM association on undamaged threshold DNA. (A) Xenopus interphase egg extracts were treated with sperm nuclei and (dA-dT)70 as indicated. The chromatin fraction was then isolated and analyzed by immunoblotting for phospho-ATM, ATM, γ-H2AX, and H2AX. (B) Xenopus interphase egg extracts were treated with sperm nuclei and (dA-dT)70 as indicated and then both (dA-dT)70 and chromatin fractions were isolated as described in Materials and Methods. γ-H2AX, H2AX, ATM, and Mre11 were measured by immunoblotting with specific antibodies. (C) As for the experiment in panel B, Xenopus interphase egg extracts were treated with sperm nuclei and (dA-dT)70, and then total chromatin was isolated by centrifugation as described in Materials and Methods. Using HRP-streptavidin blotting, the presence of (dA-dT)70 was assessed in the whole egg extract and the slot-blotted chromatin fraction. (D) Xenopus interphase egg extracts were treated with sperm nuclei and biotinylated (dA-dT)70. Oligonucleotides were then recovered with streptavidin beads as described in Materials and Methods. The presence of (dA-dT)70 in the whole egg extract and on the isolated beads was measured by HRP-streptavidin blotting. (E) Xenopus interphase egg extracts were treated with sperm nuclei, (dA-dT)70, caffeine (10 mM), and UCN-01 (0.1 μM) as indicated. Immunoblotting was then performed for phosphorylated ATM and phospho-Chk1. (F) Xenopus interphase egg extracts were treated with sperm nuclei, (dA-dT)70, caffeine, and UCN-01 as for panel E and then chromatin fractions were isolated and subjected to immunoblotting for γ-H2AX and ATM.