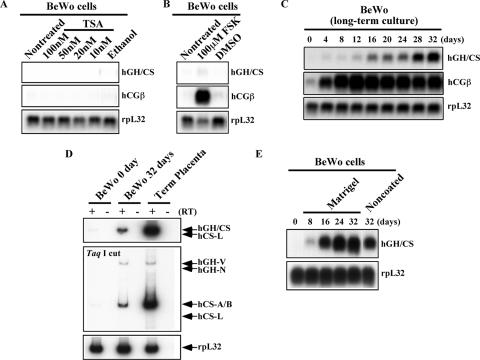
FIG. 2.
Induction kinetics of the placental hGH-hCS genes in BeWo cells. (A) Histone deacetylase inhibition with TSA failed to induce BeWo cell gene expression. The cells were treated with the indicated concentrations of TSA for 12 h. Total RNA was analyzed by Northern blotting using ethanol as a vehicle control. Hybridization probes used are indicated to the right of each panel. (B) FSK-induced BeWo cell syncytialization resulted in only a moderate increase in hGH-hCS expression. BeWo cells were induced to syncytialize by culturing them in the presence of 80 μM FSK for 4 days. Total RNA from the treated cells was used for Northern blot analyses. The syncytialization was confirmed by hCGβ induction. Dimethyl sulfoxide (DMSO) was the vehicle control. (C) Placental hGH-hCS expression was induced by long-term culture of BeWo cells. The cells were plated at a high density and incubated without chemical additives for 32 days. Total RNA (20 μg) was collected every 4 days for analysis by Northern blotting. (D) The BeWo hGH-hCS transcript profile after 32 day of culture was qualitatively similar to that in term placentas. RT-PCR analysis was performed as described for Fig. 1C but with total RNA (5 μg) from BeWo cells at days 0 and 32 of culture and from human term placental villi. hCS-A, hCS-B, and hGH-V mRNAs were detected in all three samples; no hGH-N or hCS-L signals were observed. (E) Matrigel coating of the culture plates enhanced hGH-hCS activation in BeWo cells. The BeWo cells were cultured on Matrigel-coated dishes for 32 days, and total RNA was collected every 8 days, as described for panel C above. The Northern blot analysis showed higher and faster induction of hGH-hCS genes than that shown in panel C when cells were grown on Matrigel.