Abstract
Background
Adhesive capsulitis is a common, painful, and disabling condition that has been managed with corticosteroid injections for over 50 years. There is debate over the use of single or multiple injections, but no systematic review has investigated the effects of administering multiple injections.
Aim
To assess the efficacy of treating adhesive capsulitis of the shoulder with multiple corticosteroid injections.
Design of study
Systematic review.
Method
An English language search for randomised controlled trials was conducted from: MEDLINE®, EMBASE, CINAHL, PEDro, SIGLE, National Technical Information Service, British National Bibliography, Index of Scientific and Technical Proceedings® databases, and the Cochrane Library. Randomised controlled trials were identified from reference lists of review and eligible articles. The studies were assessed using a recognised rating system of methodological trial quality. The conclusions and results of the identified studies, based on their main outcome measures, were then summarised.
Results
Nine randomised controlled trials were identified and four studies were rated as high quality. Three high quality studies showed a beneficial effect for the use of multiple corticosteroid injections with outcome measures of pain reduction, improved function, and increased range of shoulder movement.
Conclusion
The evidence suggested that multiple injections were beneficial until 16 weeks from the date of the first injection. Up to three injections were beneficial, with limited evidence that four to six injections were beneficial. No evidence was found to support giving more than six injections.
Keywords: adhesive capsulitis; injections; randomised clinical trials; review, systematic; shoulder; steroids
INTRODUCTION
Adhesive capsulitis of the shoulder is a common affliction, affecting 2–5% of the general adult population and up to 20% of patients with diabetes.1 An average general practice list of 6250 patients in England2 would expect to see 15 to 16 new cases each year.3
Painful stiffening of the shoulder was given the term ‘adhesive capsulitis’ by Neviaser in 1945.4 Codman5 used the term ‘frozen shoulder’ to describe a painful shoulder condition of insidious onset with stiffness and difficulty sleeping on the affected side. However, it was Duplay in 1872 who originaly described the condition as ‘periarthrite scapulo-humerale’.6 These terms are now used synonymously.7–9
Adhesive capsulitis is a clinical diagnosis made from a history of the gradual onset of severe shoulder pain with the progressive limitation of active and passive glenohumeral movements.8,9 The most significant loss of movement is in the external rotation of the joint.5,8
The condition is widely reported as a disease of middle age7–9 and is characterised by three phases. A painful phase, lasting between 3 and 8 months is followed by a phase of progressive stiffness or ‘adhesive phase’, typically lasting 4–6 months.8,9 The final resolution phase of gradual return of motion usually lasts 5–24 months.1,8,10
Many treatments have been advocated to treat the condition: rest, analgesia, active and passive mobilization, physiotherapy, oral and injected corticosteroids, capsular distension, manipulation under anaesthetic, and arthroscopic capsular release.7–11 Currently there is no consensus as to which is most effective.7,9 Some authors recommend a course of injections, with the number varying from one to 10.8–12 Others question the benefits of corticosteroid injections.13
The management of shoulder disorders and shoulder pain with corticosteroid injections has been the subject of several systematic reviews.13–17 No review has specifically examined the results of trials that gave multiple injections of corticosteroids for adhesive capsulitis.
METHOD
The process of this review followed the guidelines of the QUOROM statement.18 The Cochrane Library, MEDLINE®, EMBASE, CINAHL, and PEDro databases were searched from their inception up to June 2006. Searching the ‘grey literature’ to retrieve less accessible studies was undertaken using SIGLE, the National Technical Information Service, the British National Bibliography, and the Index of Scientific and Technical Proceedings®.
Searching was limited to reports in English due to lack of resources. The reference lists of publications retrieved were also reviewed to identify further studies for consideration in this review. The review strategy was to include all randomised controlled trials as they are widely viewed as the most appropriate research design for studying the effectiveness of an intervention or treatment.19,20
Key descriptors were identified from previous systematic reviews13–17 and are shown in Box 1.
Box 1. Key descriptor terms
-
▸
Shoulder pain
-
▸
Shoulder adhesive capsulitis
-
▸
Frozen shoulder
-
▸
Scapulo-humeral peri-arthritis
-
▸
Bursitis
-
▸
Injection
-
▸
Steroid
-
▸
Corticosteroid
-
▸
Adrenal cortex hormone
-
▸
Clinical trial
The inclusion criterion was a randomised controlled trial in adults with adhesive capsulitis having multiple steroid injections. Exclusion criteria were non-randomised controlled trials, shoulder conditions other than adhesive capsulitis, one or no corticosteroid injection, and patients aged <18 years, as the condition is rare in children.9
A validity assessment of the methodological quality of the studies was made using the criteria scale advocated by Van der Heijden et al in their systematic review of steroid injections for shoulder disorders.17 This scale gave an overall single quality score by combining individual items from a quality assessment tool. This scale has been used by other similar systematic reviews21,22 and it mirrored similar items to the Delphi consensus23 and the CONSORT24 quality statement for the publication of randomised controlled trials. The identified studies were assessed independently for inclusion and quality and mean scores were used to grade them.25
The scale had four main categories (study population, interventions, measurements of effects, and data presentation), which were divided into a total of 15 sections and each score points. The maximum total score was 100 with a high methodology achieving a score of more than 50. This point was chosen because the value had been used by other studies as a determination of quality (Supplementary Table 1).17,22,26
How this fits in
There are no systematic reviews on the use of multiple corticosteroid injections for adhesive capsulitis of the shoulder. There is debate over whether to give single or multiple injections. This systematic review of randomised controlled trials suggests that multiple injections were beneficial up to 16 weeks from the date of the first injection. The evidence also suggests that up to three injections were beneficial; there was limited evidence that giving up to six injections was beneficial.
Data abstraction was carried out independently. Data were analysed for the reported effect measures of reductions in pain, disability, and increase in external rotation of the shoulder joint.5,7,9,11
RESULTS
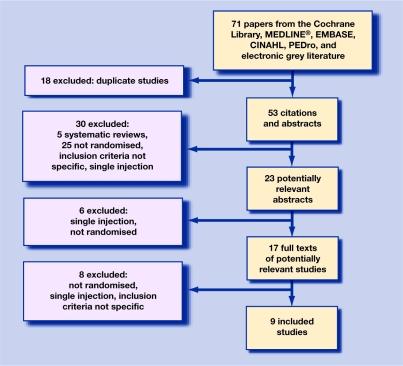
The search process identified 23 potentially relevant citations that met the search criteria. Six trials failed to meet the search criteria after reading the abstracts, as they were either not randomised or did not involve multiple injections. Ful copies of the remaining 17 trials were obtained. After analysis a further eight were excluded as they did not meet the inclusion criteria. The remaining nine studies were included in the review (Figure 1).27–35 Data from these studies were extracted onto a standardised data extraction form.36 Of these studies only four scored more than the 50 points required to indicate high methodological quality.30,33–35
Figure 1.
Process of inclusion of studies.
Population characteristics
The total number of patients included in all the studies was 476. The largest trial involved 114 patients,32 the smallest included 22 patients,34 and one trial did not provide any data on the number involved.28 Most of the studies were of small size with only two having more than 100 participants.32,35
Two studies made a power calculation,32,35 but only one of these achieved the necessary group sizes of their calculation.32 The mean age of participants showed little variation between the nine studies, with a maximum of 56 years and a minimum of 47 years of age. The sex distribution showed a considerable range between the studies (38–67% females).29,31
Settings
None of the studies were conducted in general practice in the UK. Four were conducted in UK outpatients27–30 and two were in general practice in Holland.32,35 The remaining three studies were in outpatients in Holland,33 Denmark,34 and the US.31
Inclusion and exclusion criteria
All studies used similar inclusion criteria, with the four highest-ranking studies having very similar inclusion criteria (significant loss of passive external rotation, and severe shoulder pain). However, there was not enough information reported by the studies to determine if these signs and symptoms were comparable.30,33–35
The exclusion criteria of the studies showed considerable variation: bilateral symptoms,32,33,35 diabetes,27,33–35 previous treatment,32–35 and neurological symptoms.29,31–33,35
Timescale
There was variation between the studies with a reported duration of the symptoms at baseline, ranging from a mean of 8 to 32 weeks.32,33
Treatment completion
Loss to follow-up was low apart from the study of Winters et al who lost 59% of their manipulation group and 51 % of their physiotherapy group by the end of the 11 weeks of their study.32
Comparison groups
Physiotherapy was the only intervention to be compared more than once.29,32,35 The nature of the physiotherapy itself was not directly comparable in terms of the type of treatment given, number of sessions, and the total duration of treatment.
Outcome measures
Al the studies assessed the participants pain and range of movement at the glenohumeral joint, some studies measured disability using differing scales,33,34 and one used a questionnaire.35
Pain was measured using a 10-centimetre visual analogue scale (VAS) in four studies,29,33–35 and differing scales in three studies.28,30,32 Two studies did not state how pain was assessed.27,28
Range of shoulder movement was assessed by clinical observation using different methods in four studies,27,31,32,34 and by the use of differing goniometers in four studies.29,30,33,35 The remaining study did not state how range of movement was assessed.28 Functional ability was assessed in three studies by differing methods.33–35 One study used a questionnaire35 and two used different scales.33,34 Shoulder dynamometery was assessed by a blinded observer by one study.30
The lowest scoring study failed to give any details of its methods of outcome assessment.28 There were no other common assessment methods used by the studies. Statistical comparisons were not possible between the studies because of differences in outcome assessment, duration of follow up, assessment points, and lack of data. Table 1 shows the main features of the identified studies.
Table 1.
Identified studies.
| Study | Score | Setting and numbers | Comparison groups (n) | Steroid [mg] volume used (ml) | Treatment period, weeks | Maximum number of injections | Assessment period, weeks | Changed outcome measures | Reviewers' conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Van de Windt35 | 70.5 | Dutch GP (109) | IA (53) vs physio (56) | TA [40] (1) | 6 | 3 | 52 | Symptom, pain, disability 13 weeks only | Significantly more effective at 7 and |
| De Jong33 | 59.5 | Dutch OP (57) | High dose IA (25) vs low dose IA (32) | TA [40][10] (1) | 3 | 3 | 6 | Pain, sleep, function, ROM | High dose significantly more effective than low dose |
| Jacobs30 | 55 | UK OP (50) | IA (16) vs distension with steroid (16) or air (18) | TA [40] (1/9/10) | 12 | 3 | 16 | ROM, function, pain, analgesic use | Significant improvement in pain and ROM only |
| Gam34 | 54.5 | Denmark OP (20) | IA 8 vs distension with steroid (12) | TH [20] (1/20) | 6 | 6 | 12 | Symptoms, pain, ROM, analgesic use | Significant improvement with distension in all criteria |
| Winters32 | 49.5 | Dutch GP (124) | IA SA AC (47) vs physio (35) vs manipulation (42) | TA [40] (10) | 3 | 3 | 11 | Pain, ROM | Significant improvement |
| Richardson27 | 46.5 | UK OP (101) | IA (54) vs placebo (47) | P [50] (2) | 2 | 2 | 6 | Pain, ROM | No significant improvement |
| Bulgen29 | 42 | UK OP (42) | IA and SA (11) vs mobilization (11) vs ice therapy (12) vs no treatment (8) | MP [20] (1) | 3 | 3 | 26 | Pain, ROM | No significant improvement |
| Rizk31 | 34 | US OP (48) | IA (16) or SA (16 )vs LA IA (8) or SA (8) | MP [40] (3) | 3 | 3 | 26 | Pain, ROM | No significant improvement |
| Williams28 | 15 | UK OP No data | IA vs stellate ganglion block | H [50] No data | 3 | 3 | 12 | Pain, ROM, analgesic use | Equally effective |
AC = acromoclavicular, GP = general practice, H = hydrocortisone acetate, IA = intra-articular injection, LA = local anaesthetic (lidocaine), MP = methyl prednisone acetate, OP = outpatients, physio = physiotherapy, P = prednisolone acetate, ROM = range of movement, SA = subacromial injection, TA = triamcinalone acetonide, TH = triamcinalone hexacetonide, vs = versus.
Co-interventions
Only one study33 avoided co-interventions completely. The other eight studies all differed in the co-interventions used, which reduced their external validity.
Treatment period
Single versus multiple injections
No trial compared single versus multiple corticosteroid injections. Multiple injections were compared against saline, local anaesthetic, and no treatment: one study33 compared a high dose of steroid against a low dose. The type, dosage, site (intra-articular, subacromial or acromoclavicular joint), volume of injection (1–20 ml), addition of local anaesthetic to the injection solution, and frequency of injection also varied with the studies, as did the type of local anaesthetic when used. Only two studies attempted to confirm the site of injection27,34 which was reported as ‘inconsistent’ by the study of Richardson.27
Duration of follow up
The final outcome assessment point of the studies showed considerable variation from 6 weeks after baseline33 to 52 weeks.35
Adverse reactions
Adverse reactions to corticosteroid injections were reported by four studies.30,33–35 These were increased pain after injection (10–44%), facial flushing (12.5–20%), rash (4%), and irregular menstrual bleeding (10.5%). Nineteen per cent of the side effects reported in one study33 were fever, skin irritation, sweating, fatigue, dry mouth, dizziness, and headache. No other side effects were reported.
Conclusions of the studies
The authors of the four high-methodology scoring trials concluded that steroid injections had a positive effect in their studies.30,33–35 The five highest scoring trials found that multiple injections reduced pain and/or improved movement (external rotation) from 4–16 weeks.30,32–35
From the change in outcome measures in the studies of high methodology30,33–35 there was evidence that the use of up to three corticosteroid injections was beneficial,30,33,35 limited evidence that up to six injections was beneficial,34 but no evidence for giving more than six injections to treat adhesive capsulitis.
DISCUSSION
Summary of main findings
The evidence suggests that multiple corticosteroid injections improve pain and range of motion in the short term (6–16 weeks) from first injection.30,33–35 This is of clinical value as pain and limitation of movement can often be severe. From the change in outcome measures in the studies of high methodology30,33–35 there was evidence that up to three corticosteroid injections was beneficial,30,33,35 and limited evidence that up to six injections was beneficial.34 No evidence was found for giving more than six injections to treat a single episode of adhesive capsulitis.
In terms of conservative management there was limited evidence that multiple injections were of equivalent benefit to physiotherapy in the long term of 6–12 months.35
Strengths and limitations of the study
The limitations of the review include publication bias, which may have occurred as limited resources meant that translation facilities were not available. Some trials may have been missed because they used keywords other than those identified by the search strategy, which was chosen for convenience from strategies used by reviews for answering similar, but not identical questions.13–17 The strategies used to identify the ‘grey literature’ of non-indexed and unpublished studies may not have identified all possible studies. Trials that are unpublished generally tend to have negative results, so it is important to identify this to avoid overestimation of the beneficial effects of corticosteroid injections.37 The two reviewers were not blinded, which may have caused bias in the results of the methodology scores,38 although some studies have found large discrepancies between multiple reviewers.39 A comparative quantitative presentation of the results was not possible due to differences in measurement of effects and missing or incompatible data, so the precision of the results could not be estimated.
Five different corticosteroids were used by the studies identified. Triamcinalone acetonide was the most popular.30,32,33,35 Five studies had treatment groups that combined the corticosteroid with local anaesthetic. Lidocaine was used in four studies, in volumes of 1,29 3,31 10,32 and 20 ml.34 One study used bupivacaine in 10 ml.30 All nine studies gave the injections intra-articularly. Three studies also injected the subacromial bursa,29,31,32 and one involved injection of the acromoclavicular joint.32
Only two of the studies were based in general practice and neither of these was in the UK.32,35 Caution should be placed on generalising the findings of this review to UK general practice as the location of studies can be a source of bias in systematic reviews40 and the management of shoulder pain across Europe has been reported to vary.41
No cost-effectiveness analyses were calculated by the studies. It has been reported by others that injections are cheaper and as equally effective as physiotherapy for unilateral shoulder pain in primary care.41,42
This review used a detailed assessment tool to grade the quality of the studies. However, the score or weighting of each methodological criterion remains rather arbitrary.22,43 This is important when considering weaknesses of a study that could influence its internal validity such as:
Small study size (n<40). This can exaggerate treatment effects by up to 33%.44
Duration of symptoms before treatment. Since adhesive capsulitis has three phases the studies may have been treated at differing stages of the condition.1,8–10
High drop-out rate.
The use of co-interventions.
Significance of adverse events. This may have not been fully assessed as most of the studies were of small size and short duration so not suitable to detect rare adverse events.45
Heterogeneity of the studies and the reasons for it have been analysed. As a result of this the evidence for the use of multiple injections would be suggested as a guide with need for further, more detailed, high quality studies.
Comparison with existing literature
The use of corticosteroid injections for shoulder disorders and shoulder pain has been the subject of five systematic reviews.13–17 Three of these reviews looked at shoulder disorders and shoulder pain as a whole.15–17 Partly as a consequence of their broad research questions, these reviews did not interpret their results on the basis of a specific diagnosis. The other reviews combined the results of trials that used single and multiple injections in their treatment of adhesive capsulitis and rotator cuff disease.14,16 None of these reviews specifically examined the results of trials that gave multiple injections of corticosteroids for adhesive capsulitis or the number of injections to be given and their frequency.
Implications for clinical practice and future research
This review assessed four randomised controlled trials as high quality, which justified giving multiple corticosteroid injections for adhesive capsulitis. The small sample sizes and heterogeneity of the studies meant that overall there was limited evidence to guide treatment decisions in adhesive capsulitis. There is a need for further randomised controlled trials of high methodological quality, to look a the optimum number, frequency, dose, volume, type of corticosteroid, and the importance of the anatomical site of needle placement. Better outcome to steroid injection has been reported from accurately placed injections in patients with shoulder symptoms.46,47
The adoption of a uniform definition and staging of the condition combined with a standard set of outcome measures would greatly enhance the value and generalisability of this future research.
The authors' own practice has been modified following the above evidence to inject 40 mg of triamcinalone acetonide (high dose more effective than low dose)33 with 10 ml 1% lidocaine (distension with or without corticosteroid seems beneficial)1,30,32,34 intra-articularly at intervals, increasing by 7 days each time until the patient is pain free.8,11 The normal volume of the glenohumeral joint is usually reduced to less than 10 ml in adhesive capsulitis.48 Using this method a maximum of three injections have been needed.49 Mobilizations (active and passive) can then be introduced.
Supplementary Material
Acknowledgments
Many thanks to Professor Julian Buchanan and Dr Susan Kidd for their encouragement and advice during this project. To Mrs Beryl Stanley and Mrs Frances Dowse Community Outreach Librarians, Wirral Hospital NHS Trust.
Supplementary information
Additional information accompanies this article at http://www.rcgp.org.uk/bjgp-suppinfo
Competing interests
The authors have stated that there are none
REFERENCES
- 1.Buchbinder R, Green S. Effect of arthrographic shoulder joint distension with saline and corticosteroid for adhesive capsulitis. Br J Sports Med. 2004;38(4):384–385. doi: 10.1136/bjsm.2004.013532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Royal College of General Practitioners. Profile of UK practices. 2006 May; http://www.rcgp.org.uk/information_services/information_services_home/is_publications/information_sheets.aspx (accessed 28 Jun 2007)
- 3.Van der Wint DA, Koes BW, De Jong BA. Shoulder disorders in general practice: incidence, patient characteristics and management. Ann Rheum Dis. 1995;54(12):959–964. doi: 10.1136/ard.54.12.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neviaser JS. Adhesive capsulitis of the shoulder: a study of the pathological findings in periarthritis of the shoulder. J Bone Joint Surg. 1945;27:211–222. [Google Scholar]
- 5.Codman EA. The shoulder. Boston: Thomas Told; 1934. [Google Scholar]
- 6.Duplay ES. De la périarthrite scapulo-huméral et des raideurs qui en sont la conséquence. Arch Gén Méd. 1872;20:513–542. [Google Scholar]
- 7.Dias R, Cutts S, Massoud S. Frozen shoulder. BMJ. 2005;33(7530):1453–1456. doi: 10.1136/bmj.331.7530.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ombregt L, Bisschop P, Ter Veer HJ. A system of orthopaedic medicine. 2nd edn. London: Churchill Livingstone; 2003. [Google Scholar]
- 9.Siegel LB, Cohen NJ, Gall EPG. Adhesive capsulitis: a sticky issue. Am Fam Physician. 1999;59(7):1843–1850. [PubMed] [Google Scholar]
- 10.Miller MD, Wirth MA, Rockwood CA., Jr Thawing the frozen shoulder: the ‘patient’patient. Orthopaedics. 1996;19(10):849–853. doi: 10.3928/0147-7447-19961001-06. [DOI] [PubMed] [Google Scholar]
- 11.Cyriax J. Textbook of orthopaedic medicine. 11th edn. Vol 2. London: Baillierre Tindall; 1984. [Google Scholar]
- 12.The Chartered Society of Physiotherapy. A clinical guide for the use of injection therapy by physiotherapists. London: The Chartered Society of Physiotherapy; 1999. [Google Scholar]
- 13.Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain. Cochrane Database Syst Rev. 2003;(1) doi: 10.1002/14651858.CD004016. CD004016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Speed C, Hazleman B. Shoulder pain. Clin Evid. 2003;(9):1372–1387. [PubMed] [Google Scholar]
- 15.Arroll B, Goodyear-Smith F. Corticosteroids for painful shoulder: a meta-analysis. Br J Gen Pract. 2005;55(512):224–228. [PMC free article] [PubMed] [Google Scholar]
- 16.Green S, Buchbinder R, Glazier R, Forbes A. Systematic review of randomised controlled trial of interventions for painful shoulder: selection criteria, outcome assessment and efficacy. BMJ. 1998;316(7128):354–360. doi: 10.1136/bmj.316.7128.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van der Heijden GJMG, van der Windt DA, Kleijnen J, et al. Steroid injections for shoulder disorders: a systematic review of randomised clinical trials. Br J Gen Pract. 1996;46(406):309–316. [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999;354(9193):1896–900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 19.Herbert RD, Bo K. Analysis of quality of interventions in systematic reviews. BMJ. 2005;331(7515):507–509. doi: 10.1136/bmj.331.7515.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill A, Spittlehouse C. What is critical appraisal? Oxford: Bandolier; 2001. [Google Scholar]
- 21.Koes BW, Assendelft WJJ, Van der Heijden GJMG, et al. Spinal manipulation and mobilization for back and neck pain. BMJ. 1991;303(6813):1298–1303. doi: 10.1136/bmj.303.6813.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodhead T, Clough A. A systematic review of the evidence for manipulation in the treatment of low back pain. J Ortho Med. 2005;27(3):99–121. [Google Scholar]
- 23.Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomised clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51(12):1235–1241. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357(9263):1191–1194. [PubMed] [Google Scholar]
- 25.Davies HTO, Crombie IK. What is a systematic review? http://www.evidence-based-medicine.co.uk/What_is_series.html (accessed 28 Jun 2007)
- 26.Van Tulder MW, Assendelft WJJ, et al. Method guidelines for systematic reviews in the cochrane collaboration back review group for spinal disorders. Spine. 1997;22(4):2323–2330. doi: 10.1097/00007632-199710150-00001. [DOI] [PubMed] [Google Scholar]
- 27.Richardson AT. Ernest Fletcher lecture. The painful shoulder. Proc Roy Soc Med. 1975;68(11):731–736. [PMC free article] [PubMed] [Google Scholar]
- 28.Williams NE, Seifert M, Cuddigan J, Wise R. Treatment of capsulitis of the shoulder. Rheumatol Rehabil. 1975;14(4):236. doi: 10.1093/rheumatology/14.4.236. [DOI] [PubMed] [Google Scholar]
- 29.Bulgen D, Binder A, Hazleman B, et al. Frozen shoulder: prospective clinical study with an evaluation of three treatment regimes. Ann Rheum Dis. 1984;43(3):353–360. doi: 10.1136/ard.43.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobs L, Barton M, Wallace W, et al. Intra-articular distension and steroids in the management of capsulitis of the shoulder. BMJ. 1991;302(6791):1498–1501. doi: 10.1136/bmj.302.6791.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizk T, Pinals R, Talaiver A. Corticosteroid injections andhesive capsulitis: investigation of their value and site. Arch Phys Med. 1991;72(1):20–22. [PubMed] [Google Scholar]
- 32.Winters JC, Sobel JS, Groenier KH, et al. Comparison of physiotherapy, manipulation and corticosteroid injection for treating shoulder complaints in general practice: randomised, single blind study. BMJ. 1997;314(7090):1320–1325. doi: 10.1136/bmj.314.7090.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Jong BA, Dahmen R, Hogeweg JA, Marti RK. Intra-articular triamcinolone acetonide injection in patients with capsulitis of the shoulder: a comparative study of two dose regimes. Clin Rehabil. 1998;12(3):211–215. doi: 10.1191/026921598673772420. [DOI] [PubMed] [Google Scholar]
- 34.Gam AN, Schydlowsky P, Rossel I, et al. Treatment of ‘frozen shoulder’ with distension and glucocorticoid compared with glucocorticoid alone. A randomised controlled trial. Scand J Rheumatol. 1998;27(6):425–430. doi: 10.1080/030097498442244. [DOI] [PubMed] [Google Scholar]
- 35.Van der Windt DAWM, Koes BW, Deville W, et al. Effectiveness of corticosteroid injections versus physiotherapy for treatment of painful stiff shoulder in primary care: randomised trial. BMJ. 1998;317(7168):1292–1296. doi: 10.1136/bmj.317.7168.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan KS, Ter Riet G, Glanville J, et al., editors. Undertaking systematic reviews of research on effectiveness. York: NHS Centre for Reviews and Dissemination, University of York; 2001. CRD report 4. [Google Scholar]
- 37.Hopewell S, McDonald S, Clarke M, Egger M. Grey literature in meta-analyses of randomized trials of health care interventions. The Cochrane Database of Methodology Reviews. 2002;4 doi: 10.1002/14651858.MR000010.pub3. MR000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 39.Van Tulder MW, Koes BW, Bouter LM. Conservative treatment of acute and chronic non-specific low back pain. A systematic review of randomized controlled trials of the most common interventions. Spine. 1997;22(18):2122–2156. doi: 10.1097/00007632-199709150-00012. [DOI] [PubMed] [Google Scholar]
- 40.Vickers A, Goyal N, Haland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Control Clin Trials. 1998;19(2):159–166. doi: 10.1016/s0197-2456(97)00150-5. [DOI] [PubMed] [Google Scholar]
- 41.Kassimos DG, Panayi G. Differences in the management of shoulder pain between primary and secondary care in Europe: time for a consensus. Ann Rheum Dis. 2004;63(1):111–112. [PMC free article] [PubMed] [Google Scholar]
- 42.James M, Stokes EA, Thomas E, et al. A cost consequence analysis of local corticosteroid injection and physiotherapy for the treatment of new episode of unilateral shoulder pain in primary care. Ann Rheum Dis. 2005;44(11):1447–1451. doi: 10.1093/rheumatology/kei043. [DOI] [PubMed] [Google Scholar]
- 43.Katrak P, Bialocerkowski AE, Massy-Westropp N, et al. A systematic review of the content of critical appraisal tools. doi: 10.1186/1471-2288-4-22. http://www.biomedcentral.com/1471-2288/4/22 (accessed 28 Jun 2007) [DOI] [PMC free article] [PubMed]
- 44.Moore RA, Tramer MR, Carroll D, et al. Quantitative systematic review of topically applied non-steroidal anti-inflammatory drugs. BMJ. 1998;316(7128):333–337. doi: 10.1136/bmj.316.7128.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ernst E, Pittler MH. Assessment of therapeutic safety in systematic reviews, literature review. BMJ. 2001;323(7312):546. doi: 10.1136/bmj.323.7312.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eustace JA, Brophy DP, Gibney RP, et al. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59–63. doi: 10.1136/ard.56.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308–314. [PubMed] [Google Scholar]
- 48.Neviaser RJ, Neviaser TJ. The frozen shoulder: diagnosis and management. Clin Orthop Relat Res. 1987;223:59–64. [PubMed] [Google Scholar]
- 49.Shah N. Rapid responses to: Clinical review: Frozen shoulder: Steroid injections for frozen shoulder. BMJ. 2006 http://www.bmj.com/cgi/eletters/331/7530/1453#125403 (accessed 28 Jun 2007) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.