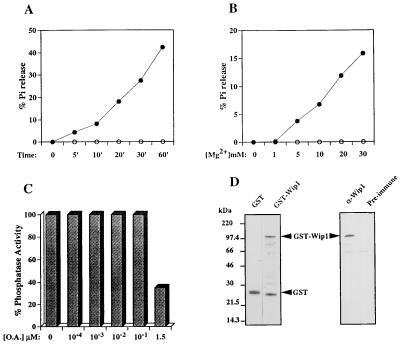
Figure 2.
Biochemical characterization of Wip1 as a PP2C. (A) A typical time course experiment showing time-dependent [32P]casein dephosphorylation in the presence of 20 mM Mg2+ by GST–Wip1 (•) but not by the control GST alone (○). PP2Cs are defined by the requirement for Mg2+ for their activity. B shows that GST–Wip1 phosphatase activity is Mg2+ concentration dependent; no activity was detected in absence of Mg2+, but increased linearly with Mg2+ concentrations ranging from 1–30 mM (incubation time 10 min). Another characteristic of the 2C class of phosphatases is their insensitivity to OA as opposed to PP1 and PP2A. (C) A GST–Wip1 phosphatase assay in presence of 20 mM Mg2+ and increasing concentrations of OA. GST–Wip1 is relatively insensitive to OA concentrations that inhibit PP1 and PP2A (10–100 nM). However, at higher concentrations (1.5 μM) GST–Wip1 activity shows partial inhibition by OA. D Left, shows a Coomassie blue-stained polyacrylamide gel of the purified GST–Wip1 and GST used in the phosphatase assays; on the right, a Western blot of the purified GST–Wip1 protein using a polyclonal antisera raised against a Wip1 carboxy-terminal peptide (GQKKIGNPLLHQRKT) (α-GST–Wip1). Preimmune sera was used as a control. Bands lower than full-length GST–Wip1 detected by Coomassie staining (Fig. 3D Left) are degradation products of the full-length protein and can be detected by Western blot analysis using polyclonal antisera raised against the GST–Wip1 fusion protein (data not shown).