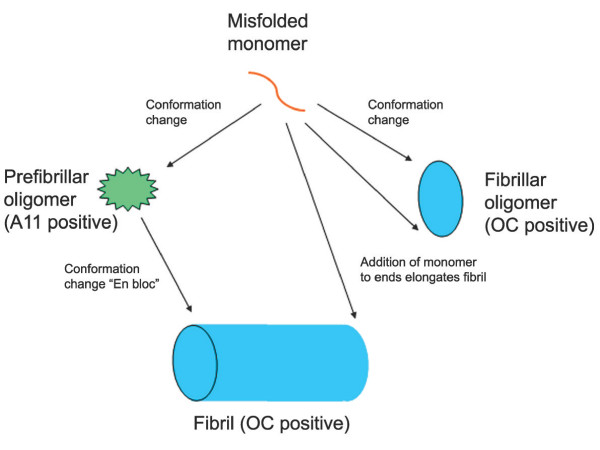
Figure 9.
Schematic representation of the distinct types of amyloid oligomers and their relationships to amyloid fibrils. Amyloid aggregation pathways begin with misfolded amyloidogenic monomer (top) and can diverge in two directions depending on which conformation it adopts. It can aggregate to form prefibrillar oligomers by adopting the conformation recognized by A11 (left pathway). These prefibrillar oligomers then align to form protofibrils (not shown) and undergo another conformation change "en bloc" to form fibrils. They are termed prefibrillar oligomers because they are transient intermediates that ultimately become fibrils. Alternatively, amyloidogenic monomer can aggregate to adopt a fibrillar conformation recognized by OC antibody (right pathway). The resulting fibrillar oligomers may represent fibril nuclei which are the minimal stable aggregate that is capable of elongating by recruiting additional monomers. Addition of monomers on to the ends of fibrillar oligomers and fibrils result in fibril growth. The distinction between fibrillar oligomers and fibrils is based on an arbitrary size difference as no conformation difference is apparent. Fibrils may be distinct from fibrillar oligomers on the basis of their content of multiple protofilaments (not shown) but this does not necessarily imply a necessary conformation difference in their integral peptide constituents.