Abstract
Enamel is formed incrementally by the secretory activity of ameloblast cells. Variable stages of secretion result in the formation of structures known as cross striations along enamel prisms, for which experimental data demonstrate a correspondence with daily periods of secretion. Patterns of variation in this daily growth are important to understanding mechanisms of tooth formation and the development of enamel thickness. Transmitted light microscopy (TLM) of histological ground sections and scanning electron microscopy (SEM) of bulk specimens or their surface replicas are the usual methods for investigating cross striations. However, these methods pose some constraints on the study of these features in Plio-Pleistocene hominid enamel, the specimens of which may only rarely be sectioned for TLM or examined on only their most superficial surfaces for SEM. The recent development of portable confocal scanning optical microscopy (PCSOM) resolves some of the restrictions on fractured enamel surfaces, allowing the visualization of cross striations by direct examination. This technology has been applied here to the study of Australopithecus africanus and Paranthropus robustus hominid molars from the Plio-Pleistocene of South Africa. We hypothesize that these taxa have increased enamel appositional rates compared with modern humans, because despite having thicker enamelled molars (particularly P. robustus), the enamel crowns of these fossil taxa take an equivalent or reduced amount of time to form. Cross striations were measured in cuspal, lateral and cervical regions of the enamel crowns, and, within each region, the inner, middle and outer zones. Values obtained for A. africanus outer zones of the enamel crown are, in general, lower than those for P. robustus, indicating faster forming enamel in the latter, while both taxa show higher rates of enamel growth than modern humans and the African great apes. This demonstrates a relatively high degree of variability in the mechanisms underlying the development of enamel across taxa.
Keywords: appositional rate, enamel development, South African hominids
Introduction
Cells known as ameloblasts, the main function of which is to secrete enamel matrix and are therefore not found in any other tissues, begin their secretory action in response to signals emanating from the odontoblasts (dentine-forming cells) (Karcher-Djuricic et al. 1985). Secretion begins at the sites of future dentine horn tips, proceeding then in the direction of the cervix (Jernvall & Thesleff, 2000). A process formed at the apical end of the cell, known as the Tomes process, is necessary for the correct development of structural units in mammalian enamel known as prisms, or rods, which are developed as by-products of secretory ameloblasts as they move from the dentine–enamel junction (DEJ) towards the outer enamel surface (OES) (Moss-Salentjin et al. 1997). In response to circadian (daily) rhythms, there is a variation in secretory activity by competent ameloblasts, which alternate between faster and slower secretory stages (Boyde, 1989). These differences in cellular behaviour between stages create structural features known as cross striations along individual enamel prisms, or rods (Boyde, 1964, 1989). Cross striations are commonly described as linear features running perpendicular to the main prism's path, or as varicosities (Dean, 1987, 2000; Boyde, 1989) (Fig. 1). Their appearance depends on the microscopic technique used, i.e. transmitted light microscopy (TLM) of ground sections, or scanning electron microscopy (SEM) of exposed internal enamel. There is structural and chemical evidence for the presence of cross striations. For instance, variations in CO3 and Na concentrations have been detected in cross striations (Boyde, 1979; Driessens et al. 1984), and Simmelink & Nygaard (1982) reported differences in porosity at the sites where cross striations appear.
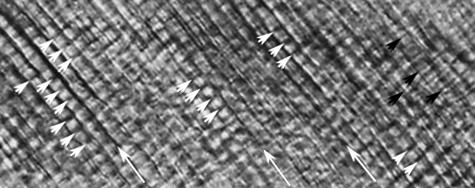
Fig. 1.
Image taken using TLM in the middle region of the cuspal enamel of a modern human molar. Cross striations or daily cell secretions are indicated by small white arrows. Other enamel features that have a shorter periodic appearance than cross striations (Dean, 2000), known as intradian lines, are here indicated by small black arrows. Large white arrows indicate the main direction of prisms. The mean cross-striation length is about 4.5 µm in this image.
Confirmation of the daily appositional growth of ameloblasts in response to the cell's circadian rhythms is based on several experimental studies dating from the 1930s onward (Schour & Poncher, 1937; Massler & Schour, 1946; Boyde, 1964; Bromage, 1991; Smith, 2006). Secretory enamel cells also periodically experience a more marked physiological disruption, which results in the development of long-term markers known as striae of Retzius (Boyde, 1989). Striae appear as more pronounced lines passing at an oblique angle to the prisms (Dean, 1987). The number of cross striations between the Retzius lines is regarded as the cross striation repeat interval, or periodicity, which in modern humans ranges from 6 to 11 days (Dean, 2000) with a mean and modal value of 9 days (Dean & Reid, 2001). In the common chimpanzee (Pan troglodytes), values range from 6 to 8 days, with an average of 7 days (Reid et al. 1998; Smith, 2004).
Enamel thickness of the permanent dentition is an important character of the adult phenotype, and features prominently in taxonomic studies within the Hominoidea (Martin, 1985; Beynon & Wood, 1986; Grine & Martin, 1988; Macho & Thackeray, 1992; Macho, 1995; Schwartz, 2000; Smith et al. 2005). The development of enamel thickness is a function of the number of active secretory cells, daily secretion rates and the timing of preprogrammed cell death (apoptosis) (e.g. Grine & Martin, 1988; Beynon et al. 1991; Macho, 1995; Dean, 2000; Dean et al. 2001). Enamel thickness, however, can be achieved via a variety of developmental pathways over given time spans and thus cannot be said to be a homologous feature across primate taxa (Dean, 1998, 2000; Schwartz, 2000; Dean et al. 2001). Therefore, an understanding of the processes involved in the development of enamel thickness is more accurately interpreted at the cellular level where variation in daily secretion rates may show differences of its development (Dean, 2000).
Some trends in daily enamel growth of primates have been recognized. In general, daily rates, or cross striation spacing, tend to increase as cells move from the DEJ toward the OES, with a corresponding decrease in appositional growth rates from the cusp to the cervical end of the tooth (Beynon et al. 1991; Reid et al. 1998; Dean, 2000; Smith, 2004; Smith et al. 2004; Ramirez Rozzi & Lacruz, in press). Interestingly, daily secretion rates are consistent between different cusps, within each region of each cusp (i.e. inner, mid and outer) and between molar types in a large sample (n = 69) of ground sections of molars of Pan troglodytes (Smith, 2004).
Daily enamel growth rates have been recorded in only a few fossil hominoids (Dean et al. 1993, 2001; Beynon et al. 1998; Schwartz et al. 2003; Smith et al. 2003, 2004). These studies are based on small sample sizes, typically one or two specimens, because the histological sectioning of fossil hominid material is not commonly permitted. To address this problem, a portable confocal scanning optical microscope (PCSOM) has been developed, which provides optical ‘sections’, equivalent to those obtained by TLM, of enamel microanatomy from the naturally fractured surfaces of bulk specimens (Bromage et al. 2005, in press). The PCSOM allows observation and measurement of cross-striation intervals and therefore provides a unique opportunity to study variation in cellular behaviour during enamel growth on relatively large samples of fossil taxa.
We have applied this technology to the study of molars of the South African hominids Australopithecus africanus and Paranthropus robustus. Both fossil taxa are characterized by thick-enamelled molars; P. robustus has ‘hyper-thick’ enamel whereas A. africanus is classified as having ‘thick’ enamel (Martin, 1985; Grine & Martin, 1988). Both taxa also have absolutely and relatively greater molar occlusal area than modern humans, and yet they appear to have formed their molars in similar or less time (Lacruz et al. in press). Given the differences in enamel thickness and the similarities in crown formation time between these taxa, our objective is to see if differences in molar thickness may in part be the result of different daily appositional rates.
There is only very limited data on cross-striation spacing (i.e. daily secretion rate) in Pliocene and early Pleistocene fossil hominid post-canine teeth; a premolar of P. boisei (Beynon & Dean, 1987) and a sample of unspecified P. boisei and early Homo molars from East Africa (Beynon & Wood, 1987) have been investigated to date. The distance between adjacent striae of Retzius was measured in A. africanus and East and South African Paranthropus molars (Beynon, 1992), and the results were then divided by a 7- and 8-day periodicity, because direct observation and measure of cross-striation spacing could not be obtained. Here we present values of cross-striation spacing, or daily appositional growth rates, in a relatively large sample of molars of A. africanus and P. robustus obtained by PCSOM. This information is compared with values recorded for modern humans and other primate taxa.
Materials and methods
We employed here a PCSOM described in detail by Bromage et al. (2003, 2005, in press). The operating principles are based on the Nipkow disc technique (Nipkow, 1884), which, in our case, employs a K2S-BIO confocal module configured to a custom stand, which facilitates vertical height adjustments. To image through long Z-height positions, the PCSOM is configured with 5×, 10×, 20× and 50× lenses, which provide working distances of 34, 19, 20 and 13 mm, respectively.
Image acquisition for the PCSOM is performed with a high-resolution 12-bit monochrome camera, which has a 2/3′ monochrome progressive scan interline CCD containing 1280 × 1024 pixels. Images are transferred in real-time to a notebook PC. PCSOM Illumination is provided by a 175-W Lambda LS xenon arc lamp, which transmits a flat and intense beam of light via a liquid light guide. The microscope returns image detail from a very thin optical plane at and immediately below the object surface (1–50 µm, depending upon specimen characteristics). To obtain two- or three-dimensional projections from a surface which is anything but perfectly flat, potential fields of view must be compiled from a through-series of captured images at all optical planes represented in the Z-axis. Images are imported into Syncroscopy Auto Montage (Syncroscopy Inc., Frederick, MD, USA), which montages only in-focus image content through a Z-series, permitting an even and fully representative image of either a pseudo-planar field of view or a three-dimensional reconstruction of surface or subsurface details.
An interesting feature of the disc design by Kino (1995) is the solution taken to suppress internal non-image-related reflections; the classic method of illuminating with polarized light to stop light reflections from within the optical system (e.g. from optical hardware within the body of the microscope), but not the useful light reflecting from the specimen and returning through the objective lens. Linear polarizing light filters and a single quarter-wave plate are employed for this purpose. The result is that all figures reported here using the PCSOM are, thus, confocal circularly polarized light images of enamel microstructure.
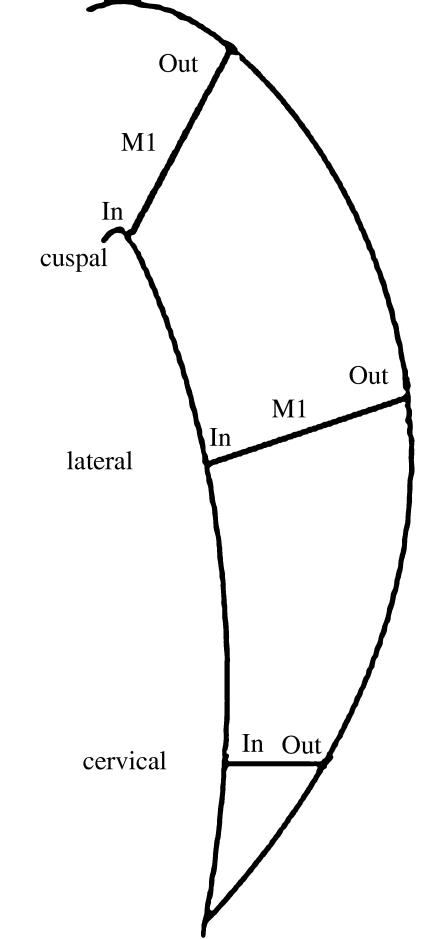
Measurements of cross-striation spacing in all teeth were taken using a measurement scheme partly based on Beynon et al. (1991), in which the enamel crown was divided into three areas corresponding to cuspal, lateral and cervical areas of each tooth (Fig. 2). At the same time, each of these areas was divided into inner, middle and outer zones, avoiding the enamel just above the cusp tips (typically characterized by ‘gnarled’ enamel). In order to include some specimens showing some degree of cuspal wear, our corresponding values for cuspal enamel were taken slightly more cervically than shown in the diagram of Beynon et al. (1991, their fig. 2C). Moreover, because it was difficult to obtain measurements near the DEJ, the values shown in inner enamel were taken no closer than 100–150 µm from the DEJ. These values may thus overestimate appositional growth in our inner enamel category when these are compared with modern taxa for which measurements were taken immediately adjacent to the DEJ.
Fig. 2.
Sketch modified from Beynon et al. (1991) indicating our measurement scheme of cross-striation spacing in fossil and modern samples used in this study.
Six molars of the South African taxa A. africanus derived from the dolomitic cave site of Sterkfontein (Member 4), and dated to about 2.5 Ma (Vrba, 1995), and seven molars of P. robustus from the sites of Swartkrans (Members 1–3) and Kromdraai B, dated at between 2.0 and 1.5 Ma (Brain, 1993; Thackeray et al. 2002), were used. Most teeth showed natural fractures developed post mortem and orientated in the occluso-cervical plane. The specimens were cleaned of any substances or matrix residue with acetone. The fractured surface of the tooth was placed approximately perpendicular to the optical axis of the PCSOM over which a drop of immersion oil and a cover slip were placed. To measure cross striations, the 50× lens was typically used with a 1 : 1 adapter; because the K2S-BIO produces a 2× magnification, this effectively results in 100× imaging conditions, providing a 190-µm field width. Measurements were taken in selected areas of the broken enamel that showed clear cross striations (Fig. 3), which in some cases were restricted to single fields of view for each division of the crown. Once these areas were identified, the distance of a minimum of 3–5 adjacent cross striations were measured in as many fields as possible. The values obtained for each group were divided by the number of cross striations measured (three or five), which yields a single representative value for cross striations at that site (Tables 1 and 2). To avoid possible discrepancies when comparing values obtained for the fossil taxa with published values for modern humans, a sample of ten modern human molars was studied using TLM of ground sections using the same measurement scheme as in the fossils. To assess differences between human and fossil hominid taxa, we employed the non-parametric Mann–Whitney U test.
Fig. 3.
Image taken with the PCSOM on the outer cervical enamel of the Paranthropus robustus specimen SK 55 from Swartkrans. Cross striations are marked with white arrows. Black arrows indicate prism direction. Image taken using a 50′ lens and 1 : 1 adapter. Scale bar = 50 µm.
Table 1.
Measurements of cross-striation spacing (µm, mean ± SD) in Paranthropus robustus and Australopithecus africanus in different regions of the cusp
| Tooth | Face | Cu. Out | Cu. Mid. | Cu. Inn | Lat. Out | Lat. Mid. | Lat. Inn | Cerv. Out | Cerv. Inn | |
|---|---|---|---|---|---|---|---|---|---|---|
| A. africanus | ||||||||||
| Stw 11 | UM3 | D/B | 6.25 ± 0.35 (10) | 5.95 ± 0.30 (9) | ? | 6.07 ± 0.49 (8) | 5.30 ± 0.34 (6) | ? | 3.95 ± 0.51 (6) | 3.75 ± 0.33 (5) |
| Stw 37 | UM3 | B/L | 6.75 ± 0.55 (9) | 5.64 ± 0.30 (7) | 4.07 ± 0.28 (5) | 5.85 ± 0.23 (6) | 5.20 ± 0.37 (8) | ? | ? | 3.70 ± 0.21 (4) |
| Stw 284 | UM2 | M/L | 6.85 ± 0.12 (7) | 5.80 ± 0.32 (8) | 4.25 ± 0.219 (6) | 6.38 ± 0.28 (9) | 5.45 ± 0.27 (7) | 4.00 ± 0.16 (9) | 4.70 ± 0.29 (5) | 3.82 ± 0.12 (4) |
| Stw 217 | UM1 | D/B | 6.90 ± 0.40 (5) | 6.18 ± 0.23 (7) | ? | 6.40 ± 0.35 (5) | 5.80 ± 0.37 (7) | 4.10 ± 0.55 (6) | 4.80 ± 0.20 (7) | 3.70 ± 0.10 (5) |
| Stw 188 | UM2 | ? | 6.30 ± 0.31 (9) | ? | ? | 6.18 ± 0.13 (7) | ? | ? | 4.40 ± 0.45 (9) | ? |
| Stw 96 | LM3 | M/B | ? | ? | ? | 5.8 ± 0.32 (9) | 4.7 ± 0.21 (7) | ? | ? | ? |
| P. robustus | ||||||||||
| SK 55 | LM2 | D/B | 7.55 ± 0.44 (11) | 6.90 ± 0.14 (9) | ? | 7.05 ± 0.58 (9) | 5.65 ± 0.12 (8) | ? | 4.62 ± 0.29 (10) | 4.01 ± 0.17 (8) |
| Skx 21841 | LM3 | M/L | 6.97 ± 0.33 (9) | 5.64 ± 0.12 (8) | 4.16 ± 0.14 (6) | 6.45 ± 0.17 (10) | 5.35 ± 0.31 (8) | 4.18 ± 0.28 (5) | 5.10 ± 0.40 (8) | 3.98 ± 0.31 (5) |
| SK 875 | frag | 7.60 ± 0.44 (7) | 6.20 ± 0.45 (6) | ? | 6.67 ± 0.32 (7) | 5.27 ± 0.16 (9) | 3.73 ± 0.21 (5) | 4.75 ± 0.53 (7) | ? | |
| SK 35 | LM2 | M/L | 6.90 ± 0.25 (8) | 5.55 ± 0.18 (7) | ? | 6.58 ± 0.22 (9) | 5.98 ± 0.23 (7) | ? | 4.40 ± 0.35 (10) | ? |
| SK 37 | LM2 | D/B | ? | ? | ? | 6.10 ± 0.12 (7) | 5.90 ± 0.23 (7) | ? | 4.95 ± 0.29 (8) | 3.70 ± 0.12 (4) |
| SKW 4771 | frag | 7.16 ± 0.51 (7) | ? | ? | ? | ? | ? | 4.80 ± 0.33 (8) | ? | |
| TM 99 | frag | ? | ? | ? | ? | ? | ? | 5.19 ± 0.55 (6) | ? | |
Tooth type and face studied are indicated. No measurements could be obtained for the areas where question marks are shown. The number in parentheses indicates the groups of cross striations measured in each region of the crown. In each group, 3–5 individual cross striations were measured.
Table 2.
Results of cross-striation measurements on a sample of ten modern human molars compared with mean values (µm, ± SD) of Paranthropus robustus and Australopithecus africanus measured using the same scheme described in the text. Modern humans show much lower rates than both hominid taxa
| Cusp. out | Cusp. mid | Cusp. Inn | Lat. out | Lat. mid. | Lat. Inn | Cerv. out | Cerv. inn | |
|---|---|---|---|---|---|---|---|---|
| H. sapiens | 5.20 ± 0.58 (10) | 4.50 ± 0.55 (10) | 2.80 ± 0.43 (10) | 4.80 ± 0.67 (10) | 4.30 ± 0.5 (10) | 2.70 ± 0.42 (10) | 3.60 ± 0.44 (9) | 2.60 ± 0.44 (10) |
| P. robustus | 7.25 ± 0.44 (5) | 6.12 ± 0.56 (4) | 4.16 (1) | 6.59 ± 0.28 (5) | 5.63 ± 0.27 (5) | 3.95 ± 0.25 (2) | 4.83 ± 0.26 (7) | 3.89 ± 0.02 (3) |
| A. africanus | 6.62 ± 0.55 (5) | 5.80 ± 0.30 (4) | 4.18 ± 0.75 (2) | 6.11 ± 0.37 (6) | 5.25 ± 0.39 (5) | 4.20 (2) | 4.46 ± 0.22 (4) | 3.74 ± 0.29 (4) |
The number in parentheses is the number of teeth included in each category for each taxon.
Results
Table 1 show values obtained for P. robustus and A. africanus, indicating the number of groups of cross striations, which on average contained 3–5 individual cross striations that were measured in each division. Table 2 contains a summary of values of cross-striation spacing obtained for the modern human sample studied here, compared with the fossil taxa, and indicating the number of teeth used in each classification. It is noteworthy that the values obtained here for modern humans using our measurement scheme are very similar to values reported in Beynon et al. (1991), except in the cervical outer area for which this study shows greater values than those previously reported. Mann–Whitney U tests reveal significant differences between modern humans and the fossil taxa in all values along the crown. Significant differences between the two fossil groups were found only in the outer cuspal area.
Discussion
The terms ‘appositional growth’ or ‘ameloblast secretion rates’ used in this work refer to two-dimensional measurements of spacing between cross striations, a practice commonly employed in the literature. However, as aptly noted by Macho et al. (2003), this measurement does not reflect the ‘true’ secretion rate of ameloblasts as the diameter of prisms is not known, and this information is vital to assess the real volume of matrix secretion. However, as pointed out by Dean (2004), it appears that prism diameter does not increase much from inner to outer enamel, and that, in general, values remain close to 5 µm (see Dean, 2004, and references therein).
The study of naturally fractured enamel has some limitations as compared with investigations based on histological sections where prisms may be followed entirely from the DEJ to the OES (Beynon et al. 1998; Dean, 1998, 2004; Dean et al. 2001). In natural fractures, prisms disappear suddenly and abrupt changes in surface topography from micrometres to millimetres occur between adjacent fields, making it difficult to follow prisms for any great length. Even then, cross striations may not always be visible in the areas where prisms remain straight, probably due to diagenetic processes. However, a substantial improvement of PCSOM over conventional light microscopy implies not having to produce a thin section as a prerequisite for excellent optical microscopy of rare and unique specimens.
The results presented here constitute a significant advance in our understanding of the variation in early hominid enamel growth mechanisms. They indicate that A. africanus and P. robustus show a similar trend of increase in growth from the inner to outer enamel areas, and a decrease from cuspal to cervical enamel (Tables 1 and 2). The Sterkfontein sample of A. africanus, which has thick enamel (but less so than P. robustus; Grine & Martin, 1988; Macho & Thackeray, 1992), has a slightly smaller cross-striation spacing in the outer enamel than P. robustus. The values reported in Beynon & Wood (1987) for P. boisei molars in mid cuspal enamel are greater than the mean obtained here for the two South African hominids in the same tooth region, although the P. boisei value falls within the range obtained here. The South African taxa show higher daily rate values than those reported for early Homo (Beynon & Wood, 1987). Beynon (1992) reported outer cuspal values in P. boisei molars that ranged from 6.9 to 7.9 µm by measuring the distance between striae and assuming a 7- or 8-day periodicity. These values are similar to those reported here for P. robustus. For A. africanus, Beynon (1992) observed values in lateral and cervical enamel that are similar to those obtained in this study for the same regions. However, some discrepancies are noticeable between his values for P. robustus and our values. The values recorded in cuspal enamel for P. robustus in Beynon (1992) are lower than the values reported here. In fact, Beynon (1992) recorded nearly constant appositional rates, ranging from about 5 to 6 µm, from cuspal to cervical enamel, which differs from our findings (Tables 1 and 2).
When daily appositional rates are compared between fossil hominids and modern humans (Risnes, 1986; Beynon et al. 1991; Dean, 1998; this study, Table 2), the extinct hominids show greater values for each given region of the cusp. In the mid and outer cuspal region, for example, the fossil taxa show values ranging from 20 to 35% higher than in H. sapiens. Comparing data available for the great apes (Beynon et al. 1991; Dean, 1998; Reid et al. 1998; Smith, 2004), we also observe that Plio-Pleistocene hominid appositional rates exceed those observed for apes in each crown division. The highest values recorded by these studies include the mean of outer cuspal enamel of Gorilla (6.1 µm, Beynon et al. 1991) and a single value recorded in a molar of H. sapiens (6.4 µm, Dean, 1998). Both values fall below the appositional rate means of the fossil taxa in the same crown region (Table 2). It is noteworthy that none of the extant primate taxa used in these studies has greater enamel thickness than either A. africanus or P. robustus.
Conclusion
Using a novel PCSOM, this study has investigated variation in daily appositional rates in broken molar enamel surfaces of P. robustus and A. africanus. This is the first study of this nature to observe variation in daily growth rates in cuspal, lateral and cervical enamel of a relatively large sample of molars of Plio-Pleistocene hominids. Given that P. robustus and A. africanus show differences in enamel thickness, but appear to have formed their molar crowns in similar time, it was expected that some differences in appositional growth would be found. In general, P. robustus shows higher outer values than A. africanus, and both fossil taxa have higher values than modern H. sapiens and the great apes. High daily secretion rates appear to be one of the mechanisms employed by both megadont fossil hominid taxa studied here to form thick enamelled molars.
Acknowledgments
The Palaeo-Anthropological Scientific Trust (PAST), D. McSherry and the L.S.B. Leakey, Blanquer and March Foundations have generously contributed financially to this research. We thank Mike Raath, Prof. P. V. Tobias, F. Thackeray and Stephany Potze for access to the material under their care. M. C. Dean, F. Ramirez Rozzi and two anonymous reviewers are thanked for providing useful comments on an earlier version of the manuscript.
References
- Beynon AD, Wood B. Variations in enamel thickness and structure in East African hominids. Am J Phys Anthropol. 1986;70:177–193. doi: 10.1002/ajpa.1330700205. [DOI] [PubMed] [Google Scholar]
- Beynon AD, Dean MC. Crown formation time of a fossil hominid premolar tooth. Arch Oral Biol. 1987;32:773–780. doi: 10.1016/0003-9969(87)90002-1. [DOI] [PubMed] [Google Scholar]
- Beynon AD, Wood B. Patterns and rates of enamel growth on the molar teeth of early hominids. Nature. 1987;326:493–496. doi: 10.1038/326493a0. [DOI] [PubMed] [Google Scholar]
- Beynon AD, Dean MC, Read DJ. On thick and thin enamel in hominoids. Am J Phys Anthropol. 1991;86:295–310. [Google Scholar]
- Beynon AD. Circaseptan rhythms in enamel development in modern humans and Plio-Pleistocene hominids. In: Smith P, Tchernov E, editors. Structure, Function and Evolution of Teeth. London: Freund; 1992. pp. 295–310. [Google Scholar]
- Beynon AD, Dean MC, Leakey MG, Reid DJ, Walker A. Comparative dental development and microstructure of Proconsul teeth from Rusinga Island, Kenya. J Hum Evol. 1998;35:163–209. doi: 10.1006/jhev.1998.0230. [DOI] [PubMed] [Google Scholar]
- Boyde A. The structure and development of mammalian enamel. University of London: 1964. PhD dissertation. [Google Scholar]
- Boyde A. Carbonate concentration, crystal centres, core dissolution, caries, cross striations, circadian rhythms and compositional contrast in SEM. J Dent Res. 1979;58b:981–983. doi: 10.1177/00220345790580025101. [DOI] [PubMed] [Google Scholar]
- Boyde A. Enamel. In: Berkovizt BKB, Boyde A, Frank RM, Hohling HJ, Moxham BJ, Nalbandian J, Tonge CH, editors. Teeth. Handbook of microscopic anatomy. Vol. 6. Berlin: Springer-Verlag; 1989. pp. 309–473. [Google Scholar]
- Brain CK. Swartkrans, a cave's chronicle of early man. Transvaal Museum Monographs. 1993;8 [Google Scholar]
- Bromage TG. Enamel incremental periodicity in the pig-tailed macaque: a polychrome fluorescent labelling study of dental hard tissues. Am J Phys Anthropol. 1991;86:205–214. [Google Scholar]
- Bromage TGA, Perez-Ochoa A, Boyde A. The Portable confocal microscope. Scanning optical microscopy anywhere. In: Méndez-Vilas A, editor. Science, Technology and Education of Microscopy: an Overview. Badajoz: Formatex; 2003. pp. 742–752. [Google Scholar]
- Bromage TG, Perez-Ochoa A, Boyde A. Portable confocal microscope reveals fossil hominid microstructure. MicroscAnal. 2005;19:5–7. [Google Scholar]
- Bromage TG, Lacruz R, Perez-Ochoa A, Boyde A. Portable confocal scanning optical microscopy of Australopithecus africanus enamel microstructure. In: Bailey S, Hublin JJ, editors. Dental Perspectives on Human Evolution. Springer: New York; 2005. in press. [Google Scholar]
- Dean MC. Growth layers and incremental markings in hard tissues, a review of the literature and some preliminary observations about enamel structure of Paranthropus boisei. J Hum Evol. 1987;16:157–172. [Google Scholar]
- Dean MC, Beynon AD, Thackeray JF, Macho GA. Histological reconstruction of dental development and age at death of a juvenile Paranthropus robustus specimen, SK 63, from Swartkrans, South Africa. Am J Phys Anthropol. 1993;91:401–419. doi: 10.1002/ajpa.1330910402. [DOI] [PubMed] [Google Scholar]
- Dean MC. A comparative study of cross striation spacing in cuspal enamel and four methods of estimating the time taken to grow molar cuspal enamel in Pan, Pongo and Homo. J Hum Evol. 1998;35:449–462. doi: 10.1006/jhev.1998.0208. [DOI] [PubMed] [Google Scholar]
- Dean MC. Progress in understanding hominoid dental development. J Anat. 2000;197:77–101. doi: 10.1046/j.1469-7580.2000.19710077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean MC, Leakey M, Reid D, et al. Growth processes in teeth distinguish modern humans from Homo erectus and earlier hominins. Nature. 2001;44:628–631. doi: 10.1038/414628a. [DOI] [PubMed] [Google Scholar]
- Dean MC, Reid DJ. Perikymata and distribution on Hominid anterior teeth. Am J Phys Anthropol. 2001;116:209–215. doi: 10.1002/ajpa.1116. [DOI] [PubMed] [Google Scholar]
- Dean MC. 2D or not 2D, and other interesting questions about enamel: reply to Macho et al. (2003) J Hum Evol. 2004;46:633–640. doi: 10.1016/j.jhevol.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Driessens FCM, Heijligers HJM, Borggreven JMPH, Woltgens JHM. Variations in the mineral composition of human enamel on the levels of cross striations and striae of Retzius. Caries Res. 1984;18:237–241. doi: 10.1159/000260771. [DOI] [PubMed] [Google Scholar]
- Grine FE, Martin LB. Enamel thickness and development in Australopithecus and Paranthropus. In: Grine FE, editor. The Evolutionary History of the Robust Australopithecines. Aldyne de Gruiter; 1988. pp. 3–42. [Google Scholar]
- Jernvall J, Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev. 2000;92:19–29. doi: 10.1016/s0925-4773(99)00322-6. [DOI] [PubMed] [Google Scholar]
- Karcher-Djuricic V, Staubli A, Meyer M, Ruch JV. Acellular dental matrices promote functional differentiation of ameloblasts. Differentiation. 1985;29:169–175. doi: 10.1111/j.1432-0436.1985.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Kino GS. Intermediate optics in Nipkow disk microscopes. In: Pawley JB, editor. Handbook of Biological Confocal Microscopy. New York: Plenum Press; 1995. pp. 155–165. [Google Scholar]
- Lacruz RS, Ramirez Rozzi F, Bromage TG. Enamel development in South African fossil hominids. J Hum Evol. in press. [DOI] [PubMed]
- Macho G, Thackeray JF. Computerized tomography and enamel thickness of maxillary molars of Plio-Pleistocene hominids from Sterkfontein, Swartkrans, and Kromdraai (South Africa): an exploratory study. J Hum Evol. 1992;89:133–143. doi: 10.1002/ajpa.1330890202. [DOI] [PubMed] [Google Scholar]
- Macho G. The significance of hominid enamel thickness for phylogenetic and life-history reconstruction. In: Moggi-Cecchi J, editor. Aspects of Dental Biology: Palaeontology, Anthropology and Evolution. Florence: International Institute for the Study of Man; 1995. pp. 51–68. [Google Scholar]
- Macho GA, Jiang Y, Spears IR. Enamel microstructure- a truly three-dimensional structure. J Hum Evol. 2003;45:81–90. doi: 10.1016/s0047-2484(03)00083-6. [DOI] [PubMed] [Google Scholar]
- Martin LB. Significance of enamel thickness in hominoid evolution. Nature. 1985;314:260–263. doi: 10.1038/314260a0. [DOI] [PubMed] [Google Scholar]
- Massler M, Schour I. The appositional life span of the enamel and dentine forming cells. J Dent Res. 1946;25:145–150. doi: 10.1177/00220345460250030601. [DOI] [PubMed] [Google Scholar]
- Moss-Salentjin L, Moss ML, Yuan MS. The ontogeny of mammalian enamel. In: Koenigswald W, Sander PM, editors. Tooth Enamel Microstructure. Proceedings of the Enamel Microstructure Workshop, University of Bonn. Rotterdam: A.A. Balkema Publishers; 1997. pp. 5–30. [Google Scholar]
- Nipkow P. Elektrisches Teleskop. 1884. Patentschrift 30105 (Kaiserliches Patentamt, Berlin), patented 06.01.1884.
- Ramirez Rozzi FV, Lacruz RS. Histological study of an upper incisor and molar of a Bonobo (Pan paniscus) individual. In: Bailey S, Hublin JJ, editors. Dental Perspectives on Human Evolution. New York: Springer; pp. 000–000. in press. [Google Scholar]
- Reid DJ, Schwartz GT, Dean C, Chandrasekera MS. A histological reconstruction of dental development in the common chimpanzee, Pan troglodytes. J Hum Evol. 1998;35:427–448. doi: 10.1006/jhev.1998.0248. [DOI] [PubMed] [Google Scholar]
- Risnes S. Enamel apposition rate and the prism periodicity in human teeth. Scand J Dent Res. 1986;94:394–404. doi: 10.1111/j.1600-0722.1986.tb01779.x. [DOI] [PubMed] [Google Scholar]
- Schour I, Poncher HG. Rate of apposition of enamel and dentine measured by effects of acute fluorosis. Am J Dis Children. 1937;54:756–757. [Google Scholar]
- Schwartz GT. Taxonomic and functional aspects of the patterning of enamel thickness distribution in extant large-bodied hominoids. Am J Phys Anthropol. 2000;111:221–244. doi: 10.1002/(SICI)1096-8644(200002)111:2<221::AID-AJPA8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Schwartz GT, Liu W, Zheng L. Preliminary investigation of dental microstructure in the Yuanmou hominoid (Lufengpithecus hudienensis), Yunnan Province, China. J Hum Evol. 2003;44:189–202. doi: 10.1016/s0047-2484(02)00197-5. [DOI] [PubMed] [Google Scholar]
- Simmelink JW, Nygaard VK. Ultrastructure of striations in carious human enamel. Caries Res. 1982;16:179–188. doi: 10.1159/000260595. [DOI] [PubMed] [Google Scholar]
- Smith TM, Martin L, Leakey MG. Enamel thickness, microstructure and development in Afropithecus turkanensis. J Hum Evol. 2003;44:283–306. doi: 10.1016/s0047-2484(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Smith TM. Incremental development of primate dental enamel. Stony Brook University: 2004. PhD thesis. [Google Scholar]
- Smith TM, Martin LB, Reid DJ, De Bonis L, Koufos GD. An examination of dental development in Graecopithecus freybergi (=Ouranopithecus macedoniensis) J Hum Evol. 2004;46:551–577. doi: 10.1016/j.jhevol.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Smith TM, Olejniczak AJ, Martin LB, Reid DJ. Variation in hominoid molar enamel thickness. J Hum Evol. 2005;48:575–592. doi: 10.1016/j.jhevol.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Smith TM. Experimental determination of the periodicity of incremental features in enamel. J Anat. 2006;208:99–103. doi: 10.1111/j.1469-7580.2006.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackeray JF, Kirschvink JL, Raub TD. Palaeomagnetic analyses of calcified deposits from the hominid site of Kromdraai, South Africa. S Afric J Sci. 2002;98:537–540. [Google Scholar]
- Vrba ES. The fossil record of African antelopes (Mammalia, Bovidae) in relation to human evolution and paleoclimate. In: Vrba ES, editor. Paleoclimate and Evolution, with Emphasis on Human Origins. New Haven, CT: Yale University Press; 1995. pp. 385–424. [Google Scholar]