Abstract
The peripheral nervous system has the intrinsic capacity to regenerate but the reinnervation of muscles is often suboptimal and results in limited recovery of function. Injuries to nerves that innervate complex organs such as the larynx are particularly difficult to treat. The many functions of the larynx have evolved through the intricate neural regulation of highly specialized laryngeal muscles. In this review, we examine the responses of nerves and muscles to injury, focusing on changes in the expression of neurotrophic factors, and highlight differences between the skeletal limb and laryngeal muscle systems. We also describe how artificial nerve conduits have become a useful tool for delivery of neurotrophic factors as therapeutic agents to promote peripheral nerve repair and might eventually be useful in the treatment of laryngeal nerve injury.
Keywords: larynx, nerve conduit, neurotrophic factor, peripheral nerve injury, synkinesis
Introduction
Peripheral nerve injury is a common problem; up to 300 000 cases present each year in Europe alone and despite surgical repair, disability often persists for the lifetime of many patients (Wiberg & Terenghi, 2003). The extent of damage determines whether or not recovery of function is possible. Seddon published his classification of nerve injuries in 1943, and Sunderland (1951) expanded this grading system to describe its clinical relevance in predicting functional outcome (reviewed in Grant et al. 1999). Neurapraxic injury involves minimal damage to axons and results in short-term conduction block. Spontaneous recovery normally occurs after several days to several weeks. In the case of axonotmesis the nerve axons are severed and nerve conduction is lost. Subsequently, a complex series of molecular and cellular reactions takes place at the lesion site in an effort to regenerate the axon for reconnection with its target organ. Given that the injury often occurs at a significant distance from the target organ, recovery of function takes months and often remains far from ideal. Neurotmesis involves damage to axons and myelin as well as the external nerve envelope layers. In this instance, the regenerative process is more disorganized with the re-growth of poor quality axons with limited function. Neurotmesis often gives rise to neuromas that prevent axons reaching their target organs. Axons may also grow in a misdirected fashion and innervate muscle fibres they did not previously contact. This process is known as synkinesis. It results in uncoordinated movement of muscles and is a particular problem for complex organs such as the larynx which require synchronized movement of many muscles for full function.
The larynx and recurrent laryngeal nerve injury
Laryngeal dysfunction occurs after injury to the recurrent laryngeal nerve (RLN). Unilateral paralysis may follow neck or cardiac surgery, or may be idiopathic and result in transient or permanent dysphonia. Bilateral paralysis is less common but more serious as it leads to life-threatening breathing complications. Causes include thyroid surgery and neurological disease such as peripheral neuritis. Current treatment options for laryngeal paralysis are far from ideal. To date, reinnervation strategies for the larynx have limited efficacy and are not a widely used treatment. Currently, static medialization techniques such as laryngeal framework surgery are performed to manage dysphonia and aspiration. In the case of bilateral damage, a tracheostomy is often required to bypass the airway obstruction, but this severely impairs quality of life and may actually aggravate aspiration. Current surgical procedures cannot restore true laryngeal motion. Thus, our group has been examining novel methods to repair the injured RLN using techniques developed for the treatment of other peripheral nerve injuries (Birchall et al. 2004; Kingham et al. 2005a).
The motor innervation of the larynx is supplied by the vagus nerve. Neuron cell bodies are located in the brainstem in the nucleus ambiguus and the retrofacial nucleus (Gacek et al. 1977). Axons destined for the RLN remain grouped with other vagal fibres until branching off into right and left RLNs in the thorax and tracking back up to the larynx. One-quarter of RLN motor axons innervate the posterior cricoarytenoid (PCA), the only laryngeal abductor muscle, which is responsible for opening the glottis upon inspiration. The remaining axons pass to the adductor muscles [thyroarytenoid (TA), lateral cricoarytenoid and interarytenoid], which act in concert with the PCA to provide fine control of phonation, swallowing and the cough reflex.
Various scenarios can occur after RLN damage. After a mild neurapraxic injury, which may occur after thyroidectomy, the nerve can regain function spontaneously after a number of weeks. By contrast, if the nerve is cut or crushed not all the regenerating axons will achieve the desired reinnervation of the laryngeal muscles. Those that do reach the muscle can prevent denervation atrophy but are unable to evoke voluntary contraction. Some axons will fail to reach their targets completely whereas others will grow in a misdirected fashion. This leads to adductor axons innervating abductor muscles and vice versa, resulting in simultaneous contraction of antagonistic muscles and mass movement (Crumley, 1989). It is because of synkinesis that surgeons have found it difficult to restore effective movement to the paralysed larynx using reinnervation techniques. Understanding changes that take place in injured peripheral nerves and the consequences of denervation for muscle function should lead to new therapies for treatment of RLN injury. Furthermore, it is important to appreciate that there may also be intrinsic differences between the responses of limb skeletal muscles and laryngeal muscles to denervation. These topics will be addressed here.
Methods for enhancing muscle reinnervation
Pharmacological and surgical strategies to enhance recovery of function aim to increase the number of appropriate axons which reach their targets in the shortest time after injury. The goal is to minimize the amount of end organ atrophy associated with denervation (Borisov et al. 2001). Loss of connectivity results in decreased trophic support for the injured neurons and increased cell death. For instance, up to 35% of the total dorsal root ganglion (DRG) sensory neuron population undergoes apoptosis following nerve transection (McKay Hart et al. 2002). Motor neurons may be less sensitive to cell death than sensory neurons (Zhang et al. 2004) but nevertheless represent a vulnerable population of cells. Neurons that survive the initial insult do so by switching on growth genes (Costigan et al. 2002) to compensate for their decreased trophic support. In this article we review the current knowledge of the changes that occur in the expression of one particular group of growth proteins, the neurotrophic factors, both at the site of nerve lesion and within the denervated muscle. Understanding how these changes influence the profile of regeneration may help in the development of pharmacological strategies to enhance neuronal survival, promote faster regeneration and ensure accurate target reinnervation.
Mechanisms of axonal regeneration
Injury to a peripheral nerve normally results in some degree of axonal regeneration and involves a complex series of cellular and molecular reactions. Wallerian degeneration leads to the sealing of severed axon ends and initiation of the regenerative phase. This results in phenotypic and morphological changes to both neurons and Schwann cells within days of injury. Axons swell as they fill with organelles and the cytoskeleton begins to break down, leading to the formation of axonal membrane fragments. This initial phase of degeneration is mediated by increased levels of intracellular calcium and activation of calcium-dependent proteases such as the calpains (George et al. 1995). Denervated Schwann cells down-regulate their expression of myelin-associated proteins and certain adhesion-dependent molecules (Gupta et al. 1988), resulting in the destruction of their myelin sheaths and the production of high levels of debris. In order for axons to re-grow across this lesion site the area must be ‘cleaned up’. Schwann cells up-regulate pro-inflammatory cytokines within hours of injury and encourage the migration of macrophages to the site of injury (Perry et al. 1987; Toews et al. 1998). Degradation of the myelin sheaths by macrophages removes growth inhibitory molecules such as myelin-associated glycoprotein (Tang et al. 1997) and encourages the proliferation of Schwann cells (Baichwal et al. 1988). Thus, within a week of injury, Schwann cells dedifferentiate and proliferate rapidly to cover the space previously filled by the degenerating axons and fragments of myelin. Furthermore, there is co-ordinated alignment of Schwann cells. When there is a gap, Schwann cells emerge from the distal stump and form columns of cells known as the bands of Bungner, which act to guide regenerating axons to the end organ.
Meanwhile, at the proximal side of the lesion, retrograde changes occur within the neuronal cell bodies. Increased lysosomal phosphatase activity after injury results in the removal of old protein-synthesizing machinery. This leads to the production of a new profile of growth-promoting proteins (Fenrich & Gordon, 2004). Axonal sprouts emerge from the proximal stump and grow towards the lesion site at a rate of 1–2 mm day−1. An excess production of sprouts ensures that at least some successfully enter the endoneurial tube and have the ability to reach their end organ. This involves a process of contact guidance between the growing axon tip and the Schwann cells lining the tube. Dedifferentiated Schwann cells up-regulate the expression of many regeneration-related genes, which produce proteins associated with axonal elongation (Hall, 2005). Thus, the ability of the peripheral nerve to regenerate is strongly determined by its level of trophic responsiveness following injury.
Altered neurotrophic factor expression in injured neurons
Understanding the changes that occur in growth factor expression in the injured peripheral nerve might indicate suitable pharmacological therapies for repair and enhancement of muscle reinnervation. In this section we review the available literature on this topic.
Nerve growth factor (NGF)
NGF was the first neurotrophic factor to be identified (Levi-Montalcini & Hamburger, 1951) and can bind to both the p75NTR and trk-A receptors (Kaplan et al. 1991). NGF is present only in low levels in the healthy nerve but shows rapid increases in expression after nerve injury (Saika et al. 1991). More recently, it has been shown that satellite glial cells surrounding the DRG up-regulate levels of NGF after axotomy and this contributes to the induction of sympathetic nerve sprouting after injury (Zhou et al. 1999). Schwann cells at the injury site can also rapidly up-regulate NGF levels, possibly to compensate for the loss of supply from the nerve itself (Abe et al. 2004). The role for NGF in recovery of motor neuron function may be limited as these cells do not express the high-affinity trk-A receptors and in vitro studies have shown little effect on neurite outgrowth in isolated neuronal cultures (Henderson et al. 1993; Wong et al. 1993). The levels of p75NTR are increased in motor neuron cell bodies and axons as well as Schwann cells during Wallerian degeneration. Two independent models of motor neuron injury using P75–/– mice showed that this up-regulation of P75 was not necessary for motor neuron survival but in fact increased the sensitivity to cell death (Ferri et al. 1998; Boyd & Gordon, 2001).
Brain-derived neurotrophic factor (BDNF)
BDNF was the second member of the neurotrophin family to be characterized (Leibrock et al. 1989). It shares 54% sequence homology with NGF and has multiple actions throughout the nervous system. The effects of BDNF are mediated principally by the tyrosine kinase receptor trk-B (Squinto et al. 1991). Auto-phosphorylation of the receptor enables it to bind and phosphorylate target proteins that affect the growth and differentiation of cells. BDNF is expressed by skeletal muscle and is an important trophic factor for motor neurons, influencing the expression of cholinergic genes and promoting cell survival in culture (Henderson et al. 1993). Evidence for a role in nerve regeneration comes from a number of in vivo studies. The levels of BDNF are up-regulated in denervated Schwann cells (Meyer et al. 1992). Blockade of BDNF with neutralizing antibodies indicates that these endogenous increases are necessary for efficient myelination and regeneration of the sciatic nerve (Zhang et al. 2000). The DRG shows increased levels of BDNF after axotomy and this may act locally to induce sprouting, as in the case of NGF (Deng et al. 2000), or it may be transported anterogradely leading to increased expression at the proximal stump (Tonra et al. 1998). The expression of trk-B has been shown to be critical for axonal regeneration (Boyd & Gordon, 2001) and enhanced levels after axotomy suggest a protective response to injury (Hammarberg et al. 2000). Furthermore, it has recently been suggested that the up-regulation of BDNF and trk-B mediates changes in the level of other regeneration-associated genes (Al-Majed et al. 2004).
Neurotrophin-3 and -4 (NT-3, NT-4)
Other proteins more distantly related to NGF are the neurotrophins, NT-3 and NT-4, each with a preferential binding pattern to trk receptors. NT-3 principally signals through the trk C receptor and is abundantly expressed in the peripheral nervous system (Katoh-Semba et al. 1996), whilst NT-4 binds to the trk-B receptor expressed by most motor neuron cells (Escandon et al. 1994). A number of in vitro and in vivo studies have indicated that both NT-3 and NT-4 are key survival factors for motor neurons (Sendtner et al. 1996). Furthermore, NT-4 has been shown to be required for the early re-growth of regenerating axons. Allografts from NT-4-knockout animals produced significantly shorter axonal extensions than wild-type animals whereas BDNF knockouts were no different (English et al. 2005).
A recent extensive study has mapped the changes in neurotrophin expression in the injured peripheral nerve after different types of injury (Omura et al. 2005). The levels of NT-3 mRNA in the sciatic nerve were significantly decreased after axotomy but not after crush injury. NT-4 mRNA was also significantly decreased after neurotmesis with fluctuating levels in the other injury paradigms. The levels of both proteins were significantly enhanced after all types of injuries. This increased production of protein is likely to come from invading inflammatory cells at or near the site of injury. For instance, enhanced levels of NT-3 have been shown to be proportional to the extent of macrophage infiltration rather than to the severity of axonal pathology (Sobue et al. 1998). Furthermore, in contrast to the anterograde transport of BDNF after injury, NT-3 is transiently up-regulated at the proximal stump and transported back to the cell body (Nitta et al. 1999).
Ciliary neurotrophic factor (CNTF)
CNTF is a 22–24-kDa neurokine protein expressed throughout the peripheral and central nervous systems and also in skeletal muscle (Sendtner et al. 1994). It binds to a glycosyl-phosphatidylinositol-linked CNTF receptor, which exhibits a high degree of homology to the α-subunit of the interleukin-6 receptor system (Grotzinger et al. 1997). In peripheral nerves, CNTF is expressed at high levels in Schwann cells but is down-regulated after injury (Smith et al. 1993; Lee et al. 1995), via the activation of a Ras extracellular-signal-regulated kinase (ERK) signalling pathway (Abe et al. 2001). The CNTF receptor is expressed by many classes of neuron but it is cranial and spinal motor neurons that are particularly sensitive to the effects of CNTF. Such neurons display a rapid, robust increase in phospho-STAT3 in their dendrites, cell bodies and nuclei upon stimulation with CNTF (MacLennan et al. 2000). Activation of STAT3 via CNTF release is an early retrograde signal in axotomized facial motor neurons (Kirsch et al. 2003). Concomitantly, the up-regulation of CNTF receptor after injury (Ito et al. 1998) is likely to be a protective response (Curtis et al. 1993). Indeed, CNTF-knockout mice show an impaired ability to recover from a sciatic nerve crush injury (Yao et al. 1999).
Glial cell line-derived neurotrophic factor (GDNF)
GDNF and its corresponding receptors are distantly related to the transforming growth factor family. GDNF promotes the survival of both motor (Henderson et al. 1994) and sensory neurons (Matheson et al. 1997) and is abundantly expressed by skeletal muscle (Nagano & Suzuki, 2003). Injury to the adult rat sciatic nerve induces rapid up-regulation of GDNF mRNA in Schwann cells at the lesion site (Hammarberg et al. 1996) and differential regulation of GNDF receptors in the sensory neuron bodies (Bennett et al. 2000). The receptors, GDNF-α and c-ret, are also increased in the facial nucleus after motor neuron axon transection (Burazin & Gundlach, 1998). GDNF stimulates the migration of Schwann cells, leading to enhanced myelination. Recent experiments have shown that GDNF activates multiple kinase proteins and utilizes cell adhesion molecule signalling pathways to regulate Schwann cell function prior to myelination (Iwase et al. 2005). Chronically denervated Schwann cells show decreased expression of GDNF and this correlates with impaired regeneration (Hoke et al. 2002).
Neuregulin-1
The neuregulin-1 family of proteins are homologous to the epidermal growth factor family and signal via the receptor tyrosine kinases of the ErbB family (reviewed in Garratt et al. 2000). A number of different laboratories discovered neuregulins independently, resulting in a complex nomenclature. Essentially the two best characterized forms of neuregulin-1 are a Type I, otherwise known as neu differentiation factor, heregulin, or acetylcholine receptor-inducing activity (ARIA), and Type II, known as glial growth factor (GGF). Type I neuregulin-1 and its associated ErbBs are concentrated at the neuromuscular junction where they regulate differentiation of muscle fibres, prevent apoptosis and modulate expression of the acetylcholine receptor gene (reviewed in Falls, 2003). Alternative splicing gives rise to various isoforms within each type. For instance, GGF-I, GGF-II and GGF-III at nanomolar concentrations have been shown to be potent mitogens for rat Schwann cells in vitro, whereas at lower concentrations they promote Schwann cell survival (Minghetti et al. 1996). Furthermore, GGF-II increases Schwann cell motility and activates Schwann cells to release factors that contribute to axonal outgrowth (Mahanthappa et al. 1996). The process of Schwann cell dedifferentiation and proliferation is a prerequisite for axonal regeneration after injury. GGF mRNA is induced in the peripheral nerve 3 days after axotomy and this coincides with the onset of Schwann cell DNA synthesis. Expression of erbB2 and erbB3 neuregulin receptors is similarly increased, suggesting that Schwann cell responses to GGF may be modulated by changes in receptor density (Carroll et al. 1997). These autocrine survival pathways help prevent significant Schwann cell apoptosis during injury (Kopp et al. 1997) and involve signalling via the mitogen-activated protein kinase pathway and activation of transcription factors (Parkinson et al. 2002).
Neurotrophic factors and recurrent laryngeal nerve injury
The majority of the above studies have focused on changes in expression of neurotrophic factors after injury to the sciatic nerve. Few studies have specifically examined this phenomenon in the RLN but it seems likely that similar changes will occur and that these could be targets for therapeutic repair. Indeed, fibroblast growth factor-2 (FGF-2) has been shown to be up-regulated in the nucleus ambiguus after RLN crush and transection injuries (Sanuki et al. 2000). Subsequent experiments showed that local administration of FGF to the injured nerve significantly reduced the morphological signs of muscle atrophy and enhanced vocal fold movement (Motoyoshi et al. 2004). Similar changes in GDNF expression in the nucleus ambiguus might also account for the effect that adenoviral GDNF gene transfer has on survival of injured motor neurons and the recovery of vocal fold movement after RLN injury (Saito et al. 2003; Araki et al. 2006).
Characteristics of skeletal and laryngeal muscles: effect of denervation
In order for a nerve to reinnervate a muscle effectively it is important to understand the changes that occur in the muscle after denervation. Motor neurons and the muscles they innervate are mutually dependent, so when a nerve is injured the muscle also undergoes degenerative and regenerative changes. Muscles contain individual muscle fibres that can be broadly classified as exhibiting a fast or slow phenotype. This classification is determined on the basis of myosin heavy chain (MyHC) protein expression and oxidative enzyme and mitochondrial content (Schiaffino & Reggiani, 1996). Thus, skeletal muscles are composed of fibres expressing slow type I (slow) or fast type II (principally including subtypes IIA, IIB and 2X) MyHC protein. Slow and type IIA fibres express high levels of oxidative enzyme, whilst type IIB fibres display an enhanced glycolytic biochemistry. Therefore, muscles which exhibit high levels of slow type protein, such as the soleus, show a low velocity of contraction and are highly resistant to fatigue. The opposite is true for fast type muscles such as the extensor digitorum longus (EDL).
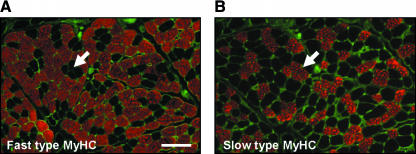
Laryngeal muscles innervated by the RLN display unique histochemical differences to limb muscle. Although these muscles express the skeletal muscle fast and slow MyHC isoforms (Fig. 1) they also contain other unique MyHC proteins that are not found in limb muscles. SDS-PAGE and Western blot analyses have shown that the TA laryngeal adductor muscle expresses a protein similar to that found in extraocular muscle (DelGaudio & Sciote, 1997), which might explain the fast contraction times of this muscle. Another characteristic marker of laryngeal muscles is the high incidence of hybrid fibres that coexpress two or more MyHCs. For instance, as many as 40% of the fibres in the human PCA are hybrids (Wu et al. 2000). Though this phenomenon is sometimes observed in skeletal muscle it is more generally associated with the process of regeneration. It has been suggested that these hybrid fibres arise because they receive frequently different patterns of neural inputs allowing the muscle to perform multiple functions.
Fig. 1.
The thyroarytenoid laryngeal adductor muscle expresses predominantly fast-type fibres. Serial transverse sections of the TA muscle immunostained for (A) fast-type MyHC (red) and (B) slow-type MyHC (red). A laminin antibody (green) was used to highlight individual muscle fibres. For clarity, arrows indicate a group of muscle fibres negative for fast- and positive for slow-type MyHC. Scale bar = 100 µm.
The electrical firing pattern of the motor neuron dictates the phenotype of individual muscle fibres (Lomo et al. 1974). When nerve injury occurs it is typical for slow muscles to become faster and fast muscles to become slower. This happens as the relative proportions of fast and slow MyHC proteins change within the muscle. For instance, in the EDL muscle there is a significant decrease in the number of IIB-positive fibres and an increase in type IIA and slow fibres after nerve transection (Michel et al. 1996; Bobinac et al. 2000). The soleus shows decreased levels of type I MyHC and undergoes de novo expression of type IIX protein (Grossman et al. 1998) after long periods of muscle inactivity. Cross-reinnervation experiments show that a fast muscle can be converted to a slow one following innervation by a slow motor nerve (Buller et al. 1960). Similarly, laryngeal muscles like limb skeletal muscle are under neural control. For instance, tonic low-frequency impulses are necessary for maintaining slow type MyHC protein expression in PCA muscle while slow fibres can be converted to fast following cross-reinnervation with the hypoglossal nerve (Paniello et al. 2001). The transitions in fast subtype proteins have been well characterized in rat RLN transection experiments. The levels of IIB MyHC protein are decreased and IIA and IIX MyHC increased (Shiotani & Flint, 1998). Furthermore, IIX MyHC protein has been shown to replace IIB proteins when examined using immunohistochemical techniques (Rhee et al. 2004). There is considerable variation in MyHC protein expression between species (reviewed in Hoh, 2005). We have investigated the MyHC protein transitions in porcine laryngeal muscles as part of our studies in developing a pig model for laryngeal transplantation (Birchall et al. 2002). When the porcine RLN is cut we find significant changes in the expression of MyHC proteins (Kingham et al. 2005a). Denervated PCA muscle exhibits decreased levels of the fast type MyHC isoforms, IIA and IIB, and increased slow type MyHC expression. Similarly, there was a fall in type IIB levels in the denervated TA muscle but increases in both IIA and slow MyHC. These relatively rapid changes in MyHC protein expression in both skeletal and laryngeal muscles precede other more obvious markers of denervation.
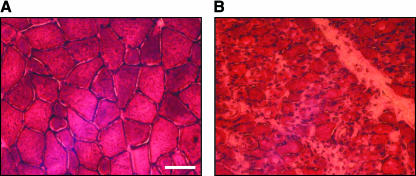
A denervated muscle undergoes a rapid decline in mass – the rat EDL muscle loses up to two-thirds of its mass within a month after denervation (Sterne et al. 1997b). This occurs as a result of individual muscle fibres undergoing atrophy, particularly in fast type fibres early in the time course of denervation. Morphological signs of denervation-induced atrophy manifest themselves as a reduction in muscle fibre diameter and the loss of the mosaic pattern of fibres normally found in control tissue (Sterne et al. 1997b). We observe similar phenomena in laryngeal muscles after RLN transection in the pig (Fig. 2). The ability of regenerating axons to make contact with muscle may also be impeded owing to the proliferation of connective tissue cells in denervated muscle. Often significant fibrosis and replacement of muscle cells with fat tissue occurs with long-term atrophy (Dulor et al. 1998).
Fig. 2.
The posterior cricoarytenoid laryngeal abductor muscle displays signs of atrophy after RLN transection. Transverse sections of (A) control PCA muscle and (B) PCA muscle 1 month after denervation. Haematoxylin and eosin staining indicates how the mosaic pattern of muscle fibres is lost and the reduction of fibre size after nerve injury. Scale bar = 50 µm.
Denervation of skeletal muscle results in the loss of one myonucleus per fibre per day (Viguie et al. 1997). Entire muscle fibres may also degenerate and die in long-term denervated muscles (Anzil & Wernig, 1989). In order to reinnervate these muscles successfully it would be necessary to find ways in which to replace these lost fibres. Indeed, the muscle initiates its own regenerative processes in an attempt to do this. Satellite cells within adult muscle act as a source of regenerating muscle fibres (Grounds et al. 2002). When a muscle is denervated it rapidly up-regulates the number of satellite cells but because there is only a limited supply, this can become exhausted in long-term denervated muscle (Viguie et al. 1997). In contrast to skeletal limb muscle, there appears to be a continuous remodelling of uninjured laryngeal muscle fibres. BrdU labelling experiments showed positive nuclei in the PCA and TA muscle but not in tibialis anterior and pectoralis major muscles (Goding et al. 2005). Other experiments, however, suggest that after denervation of laryngeal muscles there is an increase in the expression of cell cycle inhibitors such as p21 (Caiozzo et al. 2004). This might lead to an inhibition of satellite cell proliferation or result in increased apoptosis of the muscle fibres, which has been associated with the ageing TA muscle (Malmgren et al. 2001). Identifying ways in which changes in satellite cell number could be manipulated might lead to increased functional muscle reinnervation. Recent experiments have begun to elucidate the interrelationships of the myogenic response, muscle fibre atrophy and cell death mechanisms in skeletal muscle (Borisov et al. 2001).
Altered neurotrophic factor expression in denervated muscles
Changes in the expression of neurotrophic factors within the denervated muscle are likely to influence how neuromuscular connections are remodelled during peripheral nerve regeneration. These changes have been well studied in skeletal muscles.
The neurotrophin family
Denervation leads to increased NGF production by skeletal muscle 4 days after nerve injury (Amano et al. 1991). In vitro studies have shown that NGF acting through the p75NTR results in the fusion of myotubes suggesting a role in muscle differentiation (Erck et al. 1998). When myoblasts differentiate into myotubes the levels of p75NTR are down-regulated along with NGF levels (Wheeler & Bothwell, 1992). The chronic deprivation of NGF in a transgenic mouse model leads to a reduction of the size of muscle fibres in the hind-limb muscles together with an increased incidence of central nuclei indicative of muscle damage (Capsoni et al. 2000). Thus, it is tempting to speculate that NGF may play an important role in muscle maintenance and regeneration after nerve injury.
BDNF expression is up-regulated in muscle after sciatic nerve transection (Griesbeck et al. 1995). In a more detailed study, the type of nerve injury was shown to determine whether BDNF expression is altered. Neurotmesis induces rapid and sustained increases in BDNF levels in the soleus. Axotomy produces slower, transient rises, whilst crush injury is without effect (Omura et al. 2005). BDNF is expressed in both muscle fibres and the surrounding terminal Schwann cells (Meyer et al. 1992). BDNF may activate trk-B receptors found in the neuromuscular junction and has also been shown to inhibit agrin-induced clustering on cultured myotubes (Wells et al. 1999). The level of NT-3 is unaltered in the gastrocnemius and soleus muscles after sciatic nerve transection while the levels of NT-4 are increased (Funakoshi et al. 1993; Omura et al. 2005). NT-4 immunoreactivity is particularly detected in slow type muscle fibres (Funakoshi et al. 1995). Furthermore, the role of NT-4 in muscle fibre type specification has been investigated. Injection of NT-4 into the soleus muscle of neonatal rats accelerates naturally occurring transformation from fast to slow type MyHC. However, NT-4 fails to restore the normal course of this transformation in the denervated muscle, suggesting that its mechanism of action is via a retrograde signal to the motor neuron (Carrasco & English, 2003). NT-3 and NT-4 may also have diverse roles in modelling the neuromuscular junction. Muscle-derived NT-3 increases the aggregation of acetylcholine receptors in neuron–muscle co-culture (Fu et al. 1997) while NT-4 inhibits agrin-induced clustering of the receptors, the latter effect being mediated by the trk-B receptor (Wells et al. 1999).
CNTF
While muscle-derived CNTF plays an important role in motor neuron survival (Arakawa et al. 1990) it also induces sprouting at the neuromuscular junction (Siegel et al. 2000). CNTF has a myotrophic function and plays a key role in controlling protein turnover in muscle (Wang & Forsberg, 2000), regulating a number of key enzymes such as acetylcholinesterase (Boudreau-Lariviere et al. 1996). Interestingly, recent studies suggest that CNTF can also modulate the differentiation of muscle satellite cells (Chen et al. 2005) and may therefore play a role in muscle regeneration.
GDNF
GDNF plays a critical role in the development and function of synaptic connections. GDNF is constitutively supplied to the neuromuscular junction (NMJ) during postnatal development and into adulthood, suggesting its importance in maintenance of the NMJ (Nagano & Suzuki, 2003). After denervation there is an up-regulation of GDNF levels in muscle (Lie & Weis, 1998). Altered production of GDNF in muscle may be responsible for activity-dependent remodelling of the NMJ (Wehrwein et al. 2002). Over-expression of GDNF in skeletal muscle induces multiple end-plate formation and results in hyper-innervation (Zwick et al. 2001). Thus, after nerve injury, reinnervation is enhanced in myo-GDNF mice (over-expressing GDNF under the control of the myogenin promoter) but at the expense of both neurofilament integrity and functional reliability (Gillingwater et al. 2004). To date, there does not appear to be data on the effect of GDNF on muscle satellite cells.
Neurotrophic factor expression and matching of motor neurons to the muscle fibres they innervate
If a regenerating axon manages to reach a sufficiently intact muscle it should be directed to its original endplate sites. Like skeletal muscle, each laryngeal muscle fibre has a mono-neuronal innervation derived from one axon. In contrast to skeletal limb muscle, in which there is one motor end plate per muscle fibre, laryngeal axons form several connections with the muscle fibre, a phenomenon that might account for the rapid and sustained contraction properties of the laryngeal muscles (Perie et al. 1997). Numerous mechanisms during development are responsible for matching a given motor neuron to its intended target. Specificity may be defined at the level of the whole muscle, regions of the muscle or individual muscle fibre phenotype. For instance, there is evidence that a set of specific guidance cues increases the probability of slow and fast motor neurons being directed to the regions of muscle containing more slow or fast muscle fibres, respectively (Wang et al. 2002). In our own studies using nerve conduits (see below) we have shown that addition of NT-3 to nerve conduits promotes the recovery of only type IIB fibres in the gastrocnemius muscle (Sterne et al. 1997b). This led to our hypothesis that these muscle fibres are specifically innervated by a subset of trk-C-dependent motor neurons. The fact that trk-C mRNA levels are higher in type IIB-expressing EDL muscle motor neurons than soleus (slow type) motor neurons supports this hypothesis (Simon et al. 2002). Furthermore, we have shown that use of NT-3-containing conduits results in selective reinnervation of the EDL muscle (Simon et al. 2000) whilst NT-4 treatment results in improvements to the soleus muscle (Simon et al. 2003).
Neurotrophins thus represent a likely group of factors that act as guidance cues for regenerating axons, and their release at the level of the muscle might play a key role in determining the accuracy of reinnervation. Whereas skeletal muscle fibres appear to be imprinted with a unique identity during development, which enables them to express specific neurotrophins, it is unknown if the same is true for laryngeal muscles. Whereas limb muscles are derived from somites, laryngeal muscles along with other craniofacial muscles have a more complex origin (Noden, 1983). Myoblasts migrate into the branchial arches from where they give rise to individual laryngeal muscles. Studies have shown that different signalling pathways are responsible for the initiation of myogenesis in limb and craniofacial muscle development (Mootoosamy & Dietrich, 2002), suggesting there might be different patterns of muscle–motor neuron matching in the innervation of laryngeal muscles.
With a view to addressing this matter we have begun studies to determine the expression profiles of neurotrophins in laryngeal muscles after denervation (Kingham et al. 2005b). Using Western blot analysis we showed that the levels of NT-3 in the PCA muscle were significantly elevated five-fold in denervated vs. control muscles 2 months after RLN transection. The denervated TA muscle also showed a two-fold increase in NT-3 expression. The pattern of NT-4 expression followed a similar profile as NT-3. By contrast, changes in BDNF expression were only observed in the PCA muscle. There was a significant eight-fold increase in BDNF levels in the denervated PCA after 2 months. Immunohistochemistry confirmed that these changes occurred within muscle fibres themselves and not surrounding terminal glia. These results suggest that BDNF might be a specific neurotrophic factor associated with laryngeal abductor axons and could represent a suitable therapeutic agent for the reinnervation of the paralysed larynx.
Artificial nerve conduits for the delivery of neurotrophic factors
Neurotrophic factors thus have a profound effect at the neuromuscular interface, influencing both neuron survival and outgrowth and muscle cell function and regeneration. The application of these growth factors either directly to the injured nerve or intrathecally via mini-osmotic pumps has been shown to enhance peripheral nerve regeneration and in some cases promote functional muscle recovery (reviewed in Terenghi, 1999). Advanced methods of delivery might ensure some of these molecules become clinically successful therapeutic agents.
It is widely accepted that physical guidance of axons is critical for efficient nerve repair. For many years, surgeons have used autologous nerve grafts to repair large gap nerve injuries. This graft serves as a scaffold to direct regenerating axons while also providing viable Schwann cells that guide via the band of Bungner and releasing a plethora of growth factors. More recently the use of artificial nerve conduits has been suggested as an alternative to this graft as the nerve graft has a number of disadvantages. For instance, there is only a limited availability of donor material, and many lesions, such as those to the brachial plexus, require long sections of nerve. Furthermore, the requirement for a second surgical procedure results in a permanent denervation and associated sensory deficit at the donor site. The purpose of an artificial nerve conduit is to isolate the process of nerve regeneration within a tube, allowing for concentrated trophic communication between the proximal and distal nerve stumps and reduction in the infiltration of scar tissue. Furthermore, a simple tube structure can be enhanced by the addition of chemical stimulants such as neurotrophic factors and biological cues such as Schwann cells. The lumina of nerve conduits are often filled with a gel to prevent escape of axons and act as a reservoir for the added neurotrophic factors and cells.
Artificial nerve conduits may be constructed from either natural or artificial materials. Extracellular matrix (ECM) molecules such as fibronectin and collagen have been shown to influence axonal outgrowth via a number of stimulatory and inhibitory cues for growth cone migration (reviewed in Grimpe & Silver, 2002) and can be used in nerve conduits. We have compared the use of nerve repair mats constructed from orientated strands of fibronectin with autologous nerve grafts and freeze-thawed muscle grafts. Fibronectin mats supported a significantly faster rate of growth and number of axons than the freeze-thawed muscle grafts at early time points, and 15 days after repair fibronectin and nerve grafts had comparable amounts of regenerating axons and Schwann cells (Whitworth et al. 1995). Furthermore, we were able to enhance nerve regeneration further by the addition of NGF (Ahmed et al. 1999) or NT-3 (Sterne et al. 1997a) to the mats. Individual fibres of fibronectin promote Schwann cell spreading and migration prior to alignment with the axis of the fibres (Ahmed & Brown, 1999). A more recent study has shown that larger cables of fibronectin can be constructed for use in repairing longer nerve gaps. These cables provide pores of 10–200 µm that allow Schwann cells and fibroblasts to penetrate (Ahmed et al. 2003). Our work with fibronectin mats led to the discovery that treatment with neurotrophins has selective effects on muscle reinnervation as previously described.
A number of collagen-based nerve conduits has also been successfully used to repair experimental nerve gaps (Archibald et al. 1991; Kitahara et al. 1999) but repair across large gaps is limited (Yoshii & Oka, 2001). Cross-linking BDNF to the tubes produces favourable recovery of function of limb muscles and the largest mean axon diameters proximal and distal to the repair site (Utley et al. 1996). In a later experiment by the same group it was shown that addition of CNTF to the BDNF collagen tubes resulted in more enhanced recovery (Ho et al. 1998). Addition of collagen gels to the lumina of nerve conduits speeds the rate of nerve regeneration (Labrador et al. 1998). Collagen gel can also be used to deliver growth factors effectively. NT-3 and BDNF enhance peripheral nerve regeneration through synthetic tubes supplemented with collagen gel (Midha et al. 2003).
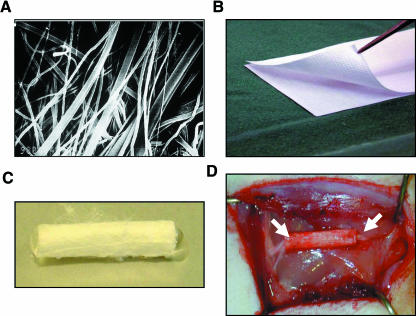
Because natural polymers such as the ECM molecules are biocompatible and have few toxic side-effects, many other similar molecules have been investigated for peripheral nerve repair. Our own recent studies have focused on poly-3-hydroxybutyrate (PHB). PHB is a member of the polyhydroxyalkanoate family of polyesters, which are considered to be attractive tissue engineering materials (reviewed in Chen & Wu, 2005). PHB was discovered as a storage product of bacteria but it is now known that it plays important physiological functions in a wide variety of cells (Reusch, 1995). We showed that PHB manufactured as bioresorbable sheets (Fig. 3) could be used in primary nerve repair without evoking an inhibitory inflammatory response (Hazari et al. 1999b). Subsequent experiments showed that PHB could be used to bridge a 10-mm nerve gap. There was a progressive increase in axon density and Schwann cell number up to 30 days after repair, which compared favourably with the changes occurring in an autograft control (Hazari et al. 1999a). Furthermore, PHB permitted good angiogenesis, a prerequisite for successful regeneration. LacZ transduced Schwann cells were then transplanted into the conduit. After 3 weeks, transplanted Schwann cells were clearly identified taking part in the regeneration process and enhanced the axonal regeneration rate by 100% (at the optimal concentration) compared with empty conduits (Mosahebi et al. 2001). Further experiments compared the effects of allogeneic vs. syngeneic Schwann cell transplantation (Mosahebi et al. 2002). Although allogeneic Schwann cells were rejected at 6 weeks, both groups equally enhanced the axonal regeneration distance, though the quantity of axons was greater using syngeneic cells. The penetration of allogeneic Schwann cells into the conduits was similar to that of the syngeneic Schwann cells, indicating the absence of any deleterious immune response. Therefore, a PHB conduit containing allogeneic Schwann cells may have a useful clinical application. In order to deliver the Schwann cells efficiently within the conduit we have subsequently used alginate hydrogels as a matrix material to fill the lumen of the tubes (Mosahebi et al. 2003). This compound has also proved useful in the delivery of neurotrophic factors within the conduit. GGF was mixed in alginate gel and injected into a PHB conduit, which was then used to repair either a 2- or a 4-cm nerve gap (Mohanna et al. 2005). PHB–GGF conduits significantly increased the quantity of Schwann cell and axonal regeneration compared with those in control conduits as well as significantly reducing the loss in muscle mass observed 4 months after nerve transection.
Fig. 3.
Poly-3-hydroxybutyrate artificial nerve conduits for peripheral nerve repair. (A) Individual PHB fibres shown under a scanning microscope can be manufactured into sheets (B), which contain fibres aligned in an identical orientation. PHB sheets are then either wrapped around the injured nerve ends or formed into tubular structures (C) to enclose the nerve (D) (arrows indicate sutured ends of nerve), to provide an environment conducive for regeneration.
Many research groups are actively investigating synthetic materials for peripheral nerve repair. Hudson et al. (1999) reviewed the other properties that materials should fulfil in order to produce a successful nerve conduit. One important factor highlighted is that the material should have the ability to deliver growth factors in a controlled manner. Polyesters such as polyglyolic acid (PGA), polylactic acid (PLA) and poly(lactic-coglycolic) acid (PLGA) have been the most commonly researched materials because of their wide availability, ease with which they can be modified and early regulatory approval for other applications (Hudson et al. 1999). Growth factors have been used successfully in these conduits. For instance, GGF has been delivered directly to the lesion using PLGA conduits (Bryan et al. 2000). GGF conduits enhanced the total number of axons and significantly increased the number of blood vessels compared with saline controls. Whilst combining GGF with Schwann cells negated the enhanced numbers of axons and blood vessels seen with GGF alone, this combination resulted in the highest myelination index and the fastest conduction velocities recorded. Microspheres formed from PLGA have been used to encapsulate neurotrophic factors (Mittal et al. 1994) and these might prove useful for the slow continual release of growth factors directly to the lesion. A search of the literature suggests that the number of synthetic materials under research for peripheral nerve injury continues to expand (Pego et al. 2001; Sundback et al. 2005; Ciardelli & Chiono, 2006). The ability of these materials to deliver growth factors will be an important factor in determining if they can enhance recovery of function.
Neurotrophic factors and nerve conduits for RLN repair and laryngeal muscle reinnervation
Because synkinesis represents a major barrier to direct repair of the RLN, there has been limited success in promoting accurate laryngeal muscle reinnervation after nerve injury. Laryngeal pacemakers have been used to provide short-term function and maintenance of muscle mass (the so called ‘baby-sitting’ effect) while nerve regeneration proceeds (Zealear et al. 2003) but these systems are still far from ideal and do not affect the process of regeneration itself. Anatomical reinnervation provides an alternative approach to this. Given that the PCA muscle is critical for the breathing functions of the larynx most studies have focused on ways to reinnervate this muscle. The phrenic nerve can be re-routed to innervate the PCA and drive laryngeal abduction (Fex, 1970; Crumley, 1983). Nevertheless, aberrant reinnervation can still occur in this system. We have therefore sought ways to guide regenerating phrenic nerve axons more accurately and speed the processes of regeneration and muscle reinnervation.
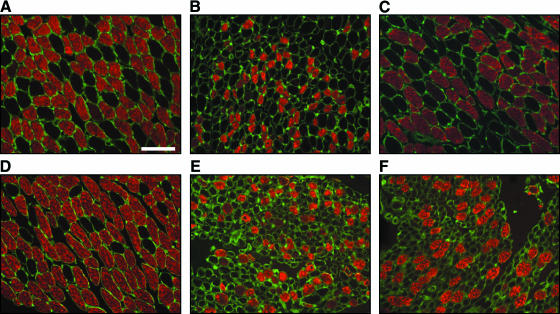
We have built on our previous work with the use of PHB nerve conduits for peripheral nerve repair. Using a pig model we performed unilateral RLN transection followed by anastomosis of the phrenic and RLN repaired with PHB mats (Birchall et al. 2004; Kingham et al. 2005a). The aim was to reinnervate the PCA muscle selectively; therefore, the adductor branches of the RLN at the point of entry to the larynx were cut and tied to prevent regeneration. There was a progressive and organized regeneration of phrenic nerve axons across the conduit, 1–4 months after injury, which led to some recovery of function of the PCA at 4 months. Analysis of the laryngeal muscles revealed that the diameter of the fast fibres was reduced after denervation (Birchall et al. 2004). The size of the PCA muscle fibres tended to increase after repair, suggesting that they were progressively reinnervated (Fig. 4). Analysis of the content of MyHC protein revealed there were also significant changes in the isoforms expressed after denervation (Kingham et al. 2005a). Overall there was a decline in fast-type MyHC proteins and an increase in slow-type MyHC. Four months after nerve repair, the levels of fast- and slow-type MyHC protein in the PCA were not significantly different from control, suggesting that the muscle had regained its normal motor neuron innervation pattern. By contrast, the levels of MyHC in the TA remained abnormal, indicating that this muscle was denervated and our method of RLN repair using conduits promoted accurate reinnervation of the PCA muscle.
Fig. 4.
Laryngeal muscle fibre morphology after RLN transection. Transverse sections of the posterior cricoarytenoid (top row) and thyroarytenoid (bottom row) muscles immunostained for fast-type MyHC (red) and laminin (green) to highlight individual muscle fibres. A unilateral phrenic–PCA abductor branch anastomosis was performed with repair using PHB nerve conduits (Birchall et al. 2004). Two months after surgery both muscles on the operated side (B and E) showed reduced muscle fibre diameter and expression of fast-type MyHC protein compared with the control side (A and D). After 4 months the PCA muscle fibre diameter appeared similar to control with increased expression of fast-type MyHC protein (C). By contrast, the TA consisted of many small-diameter muscle fibres indicating an atrophic state (F). These results suggest accurate, specific reinnervation of the PCA muscle only. Scale bar = 100 µm.
These results are highly encouraging but remain suboptimal. We are therefore continuing our studies of the relationship between laryngeal muscle phenotype, motor neuron innervation pathways and neurotrophic factor expression. This should enable us to select a suitable growth factor for addition to the nerve conduit and, we hope, enhance laryngeal muscle reinnervation and speed recovery of function.
Acknowledgments
Work from this laboratory on laryngeal muscles is supported by the Wellcome Trust.
References
- Abe K, Namikawa K, Honma M, et al. Inhibition of Ras extracellular-signal-regulated kinase (ERK) mediated signaling promotes ciliary neurotrophic factor (CNTF) expression in Schwann cells. J Neurochem. 2001;77:700–703. doi: 10.1046/j.1471-4159.2001.00286.x. [DOI] [PubMed] [Google Scholar]
- Abe S, Mizusawa I, Kanno K, et al. Induction of nerve growth factor mRNA in a rat dorsal root ganglion after application of a tourniquet. Acta Neuropathol (Berl) 2004;108:183–188. doi: 10.1007/s00401-004-0870-y. [DOI] [PubMed] [Google Scholar]
- Ahmed Z, Brown RA. Adhesion, alignment, and migration of cultured Schwann cells on ultrathin fibronectin fibres. Cell Motil Cytoskeleton. 1999;42:331–343. doi: 10.1002/(SICI)1097-0169(1999)42:4<331::AID-CM6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Ahmed Z, Brown RA, Ljungberg C, Wiberg M, Terenghi G. Nerve growth factor enhances peripheral nerve regeneration in non-human primates. Scand J Plast Reconstr Surg Hand Surg. 1999;33:393–401. doi: 10.1080/02844319950159091. [DOI] [PubMed] [Google Scholar]
- Ahmed Z, Underwood S, Brown RA. Nerve guide material made from fibronectin: assessment of in vitro properties. Tissue Eng. 2003;9:219–231. doi: 10.1089/107632703764664693. [DOI] [PubMed] [Google Scholar]
- Al-Majed AA, Tam SL, Gordon T. Electrical stimulation accelerates and enhances expression of regeneration-associated genes in regenerating rat femoral motoneurons. Cell Mol Neurobiol. 2004;24:379–402. doi: 10.1023/B:CEMN.0000022770.66463.f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, Yamakuni T, Okabe N, Sakimura K, Takahashi Y. Production of nerve growth factor in rat skeletal muscle. Neurosci Lett. 1991;132:5–7. doi: 10.1016/0304-3940(91)90418-s. [DOI] [PubMed] [Google Scholar]
- Anzil AP, Wernig A. Muscle fibre loss and reinnervation after long-term denervation. J Neurocytol. 1989;18:833–845. doi: 10.1007/BF01187235. [DOI] [PubMed] [Google Scholar]
- Arakawa Y, Sendtner M, Thoenen H. Survival effect of ciliary neurotrophic factor (CNTF) on chick embryonic motoneurons in culture: comparison with other neurotrophic factors and cytokines. J Neurosci. 1990;10:3507–3515. doi: 10.1523/JNEUROSCI.10-11-03507.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K, Shiotani A, Watabe K, Saito K, Moro K, Ogawa K. Adenoviral GDNF gene transfer enhances neurofunctional recovery after recurrent laryngeal nerve injury. Gene Ther. 2006;13:296–303. doi: 10.1038/sj.gt.3302665. [DOI] [PubMed] [Google Scholar]
- Archibald SJ, Krarup C, Shefner J, Li ST, Madison RD. A collagen-based nerve guide conduit for peripheral nerve repair: an electrophysiological study of nerve regeneration in rodents and nonhuman primates. J Comp Neurol. 1991;306:685–696. doi: 10.1002/cne.903060410. [DOI] [PubMed] [Google Scholar]
- Baichwal RR, Bigbee JW, DeVries GH. Macrophage-mediated myelin-related mitogenic factor for cultured Schwann cells. Proc Natl Acad Sci USA. 1988;85:1701–1705. doi: 10.1073/pnas.85.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DL, Boucher TJ, Armanini MP, et al. The glial cell line-derived neurotrophic factor family receptor components are differentially regulated within sensory neurons after nerve injury. J Neurosci. 2000;20:427–437. doi: 10.1523/JNEUROSCI.20-01-00427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchall MA, Bailey M, Barker EV, Rothkotter HJ, Otto K, Macchiarini P. Model for experimental revascularized laryngeal allotransplantation. Br J Surg. 2002;89:1470–1475. doi: 10.1046/j.1365-2168.2002.02234.x. [DOI] [PubMed] [Google Scholar]
- Birchall M, Idowu B, Murison P, et al. Laryngeal abductor muscle reinnervation in a pig model. Acta Otolaryngol. 2004;124:839–846. doi: 10.1080/00016480410022507. [DOI] [PubMed] [Google Scholar]
- Bobinac D, Malnar-Dragojevic D, Bajek S, Soic-Vranic T, Jerkovic R. Muscle fiber type composition and morphometric properties of denervated rat extensor digitorum longus muscle. Croat Med J. 2000;41:294–297. [PubMed] [Google Scholar]
- Borisov AB, Dedkov EI, Carlson BM. Interrelations of myogenic response, progressive atrophy of muscle fibers, and cell death in denervated skeletal muscle. Anat Rec. 2001;264:203–218. doi: 10.1002/ar.1155. [DOI] [PubMed] [Google Scholar]
- Boudreau-Lariviere C, Sveistrup H, Parry DJ, Jasmin BJ. Ciliary neurotrophic factor: regulation of acetylcholinesterase in skeletal muscle and distribution of messenger RNA encoding its receptor in synaptic versus extrasynaptic compartments. Neuroscience. 1996;73:613–622. doi: 10.1016/0306-4522(96)00033-4. [DOI] [PubMed] [Google Scholar]
- Boyd JG, Gordon T. The neurotrophin receptors, trkB and p75, differentially regulate motor axonal regeneration. J Neurobiol. 2001;49:314–325. doi: 10.1002/neu.10013. [DOI] [PubMed] [Google Scholar]
- Bryan DJ, Holway AH, Wang KK, et al. Influence of glial growth factor and Schwann cells in a bioresorbable guidance channel on peripheral nerve regeneration. Tissue Eng. 2000;6:129–138. doi: 10.1089/107632700320757. [DOI] [PubMed] [Google Scholar]
- Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burazin TC, Gundlach AL. Up-regulation of GDNFR-alpha and c-ret mRNA in facial motor neurons following facial nerve injury in the rat. Brain Res Mol Brain Res. 1998;55:331–336. doi: 10.1016/s0169-328x(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Caiozzo VJ, Wu YZ, Baker MJ, Crumley R. Effects of denervation on cell cycle control in laryngeal muscle. Arch Otolaryngol Head Neck Surg. 2004;130:1056–1068. doi: 10.1001/archotol.130.9.1056. [DOI] [PubMed] [Google Scholar]
- Capsoni S, Ruberti F, Di Daniel E, Cattaneo A. Muscular dystrophy in adult and aged anti-NGF transgenic mice resembles an inclusion body myopathy. J Neurosci Res. 2000;59:553–560. doi: 10.1002/(SICI)1097-4547(20000215)59:4<553::AID-JNR11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Carrasco DI, English AW. Neurotrophin 4/5 is required for the normal development of the slow muscle fiber phenotype in the rat soleus. J Exp Biol. 2003;206:2191–2200. doi: 10.1242/jeb.00412. [DOI] [PubMed] [Google Scholar]
- Carroll SL, Miller ML, Frohnert PW, Kim SS, Corbett JA. Expression of neuregulins and their putative receptors, ErbB2 and ErbB3, is induced during Wallerian degeneration. J Neurosci. 1997;17:1642–1659. doi: 10.1523/JNEUROSCI.17-05-01642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GQ, Wu Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials. 2005;26:6565–6578. doi: 10.1016/j.biomaterials.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Chen X, Mao Z, Liu S, et al. Dedifferentiation of adult human myoblasts induced by ciliary neurotrophic factor in vitro. Mol Biol Cell. 2005;16:3140–3151. doi: 10.1091/mbc.E05-03-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardelli G, Chiono V. Materials for peripheral nerve regeneration. Macromol Biosci. 2006;6:13–26. doi: 10.1002/mabi.200500151. [DOI] [PubMed] [Google Scholar]
- Costigan M, Befort K, Karchewski L, et al. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumley RL. Phrenic nerve graft for bilateral vocal cord paralysis. Laryngoscope. 1983;93:425–428. doi: 10.1002/lary.1983.93.4.425. [DOI] [PubMed] [Google Scholar]
- Crumley RL. Laryngeal synkinesis: its significance to the laryngologist. Ann Otol Rhinol Laryngol. 1989;98:87–92. doi: 10.1177/000348948909800201. [DOI] [PubMed] [Google Scholar]
- Curtis R, Adryan KM, Zhu Y, Harkness PJ, Lindsay RM, DiStefano PS. Retrograde axonal transport of ciliary neurotrophic factor is increased by peripheral nerve injury. Nature. 1993;365:253–255. doi: 10.1038/365253a0. [DOI] [PubMed] [Google Scholar]
- Del Gaudio JM, Sciote JJ. Changes in myosin expression in denervated laryngeal muscle. Ann Otol Rhinol Laryngol. 1997;106:1076–1081. doi: 10.1177/000348949710601212. [DOI] [PubMed] [Google Scholar]
- Deng YS, Zhong JH, Zhou XF. BDNF is involved in sympathetic sprouting in the dorsal root ganglia following peripheral nerve injury in rats. Neurotox Res. 2000;1:311–322. doi: 10.1007/BF03033260. [DOI] [PubMed] [Google Scholar]
- Dulor JP, Cambon B, Vigneron P, et al. Expression of specific white adipose tissue genes in denervation-induced skeletal muscle fatty degeneration. FEBS Lett. 1998;439:89–92. doi: 10.1016/s0014-5793(98)01216-2. [DOI] [PubMed] [Google Scholar]
- English AW, Meador W, Carrasco DI. Neurotrophin-4/5 is required for the early growth of regenerating axons in peripheral nerves. Eur J Neurosci. 2005;21:2624–2634. doi: 10.1111/j.1460-9568.2005.04124.x. [DOI] [PubMed] [Google Scholar]
- Erck C, Meisinger C, Grothe C, Seidl K. Regulation of nerve growth factor and its low-affinity receptor (p75NTR) during myogenic differentiation. J Cell Physiol. 1998;176:22–31. doi: 10.1002/(SICI)1097-4652(199807)176:1<22::AID-JCP3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Escandon E, Soppet D, Rosenthal A, et al. Regulation of neurotrophin receptor expression during embryonic and postnatal development. J Neurosci. 1994;14:2054–2068. doi: 10.1523/JNEUROSCI.14-04-02054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls DL. Neuregulins and the neuromuscular system: 10 years of answers and questions. J Neurocytol. 2003;32:619–647. doi: 10.1023/B:NEUR.0000020614.83883.be. [DOI] [PubMed] [Google Scholar]
- Fenrich K, Gordon T. Canadian Association of Neuroscience review: axonal regeneration in the peripheral and central nervous systems – current issues and advances. Can J Neurol Sci. 2004;31:142–156. doi: 10.1017/s0317167100053798. [DOI] [PubMed] [Google Scholar]
- Ferri CC, Moore FA, Bisby MA. Effects of facial nerve injury on mouse motoneurons lacking the p75 low-affinity neurotrophin receptor. J Neurobiol. 1998;34:1–9. [PubMed] [Google Scholar]
- Fex S. Functioning remobilization of vocal cords in cats with permanent recurrent laryngeal nerve paresis. Acta Otolaryngol. 1970;69:294–301. doi: 10.3109/00016487009123367. [DOI] [PubMed] [Google Scholar]
- Fu AK, Ip FC, Lai KO, Tsim KW, Ip NY. Muscle-derived neurotrophin-3 increases the aggregation of acetylcholine receptors in neuron-muscle co-cultures. Neuroreport. 1997;8:3895–3900. doi: 10.1097/00001756-199712220-00011. [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Frisen J, Barbany G, et al. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol. 1993;123:455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi H, Belluardo N, Arenas E, et al. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science. 1995;268:1495–1499. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- Gacek RR, Malmgren LT, Lyon MJ. Localization of adductor and abductor motor nerve fibers to the larynx. Ann Otol Rhinol Laryngol. 1977;86:771–776. [PubMed] [Google Scholar]
- Garratt AN, Britsch S, Birchmeier C. Neuregulin, a factor with many functions in the life of a schwann cell. Bioessays. 2000;22:987–996. doi: 10.1002/1521-1878(200011)22:11<987::AID-BIES5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- George EB, Glass JD, Griffin JW. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J Neurosci. 1995;15:6445–6452. doi: 10.1523/JNEUROSCI.15-10-06445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingwater TH, Thomson D, Ribchester RR. Myo-GDNF increases non-functional polyinnervation of reinnervated mouse muscle. Neuroreport. 2004;15:21–25. doi: 10.1097/00001756-200401190-00006. [DOI] [PubMed] [Google Scholar]
- Goding GS, Jr, Al-Sharif KI, McLoon LK. Myonuclear addition to uninjured laryngeal myofibers in adult rabbits. Ann Otol Rhinol Laryngol. 2005;114:552–557. doi: 10.1177/000348940511400711. [DOI] [PubMed] [Google Scholar]
- Grant GA, Goodkin R, Kliot M. Evaluation and surgical management of peripheral nerve problems. Neurosurgery. 1999;44:825–839. doi: 10.1097/00006123-199904000-00077. discussion 839–840. [DOI] [PubMed] [Google Scholar]
- Griesbeck O, Parsadanian AS, Sendtner M, Thoenen H. Expression of neurotrophins in skeletal muscle: quantitative comparison and significance for motoneuron survival and maintenance of function. J Neurosci Res. 1995;42:21–33. doi: 10.1002/jnr.490420104. [DOI] [PubMed] [Google Scholar]
- Grimpe B, Silver J. The extracellular matrix in axon regeneration. Prog Brain Res. 2002;137:333–349. doi: 10.1016/s0079-6123(02)37025-0. [DOI] [PubMed] [Google Scholar]
- Grossman EJ, Roy RR, Talmadge RJ, Zhong H, Edgerton VR. Effects of inactivity on myosin heavy chain composition and size of rat soleus fibers. Muscle Nerve. 1998;21:375–389. doi: 10.1002/(sici)1097-4598(199803)21:3<375::aid-mus12>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Grotzinger J, Kurapkat G, Wollmer A, Kalai M, Rose-John S. The family of the IL-6-type cytokines: specificity and promiscuity of the receptor complexes. Proteins. 1997;27:96–109. [PubMed] [Google Scholar]
- Grounds MD, White JD, Rosenthal N, Bogoyevitch MA. The role of stem cells in skeletal and cardiac muscle repair. J Histochem Cytochem. 2002;50:589–610. doi: 10.1177/002215540205000501. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Poduslo JF, Mezei C. Temporal changes in PO and MBP gene expression after crush-injury of the adult peripheral nerve. Brain Res. 1988;464:133–141. doi: 10.1016/0169-328x(88)90005-8. [DOI] [PubMed] [Google Scholar]
- Hall S. The response to injury in the peripheral nervous system. J Bone Joint Surg Br. 2005;87:1309–1319. doi: 10.1302/0301-620X.87B10.16700. [DOI] [PubMed] [Google Scholar]
- Hammarberg H, Piehl F, Cullheim S, Fjell J, Hokfelt T, Fried K. GDNF mRNA in Schwann cells and DRG satellite cells after chronic sciatic nerve injury. Neuroreport. 1996;7:857–860. doi: 10.1097/00001756-199603220-00004. [DOI] [PubMed] [Google Scholar]
- Hammarberg H, Piehl F, Risling M, Cullheim S. Differential regulation of trophic factor receptor mRNAs in spinal motoneurons after sciatic nerve transection and ventral root avulsion in the rat. J Comp Neurol. 2000;426:587–601. doi: 10.1002/1096-9861(20001030)426:4<587::aid-cne7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Hazari A, Wiberg M, Johansson-Ruden G, Green C, Terenghi G. A resorbable nerve conduit as an alternative to nerve autograft in nerve gap repair. Br J Plast Surg. 1999a;52:653–657. doi: 10.1054/bjps.1999.3184. [DOI] [PubMed] [Google Scholar]
- Hazari A, Johansson-Ruden G, Junemo-Bostrom K, et al. A new resorbable wrap-around implant as an alternative nerve repair technique. J Hand Surg [Br] 1999b;24:291–295. doi: 10.1054/jhsb.1998.0001. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Camu W, Mettling C, et al. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature. 1993;363:266–270. doi: 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, et al. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Ho PR, Coan GM, Cheng ET, et al. Repair with collagen tubules linked with brain-derived neurotrophic factor and ciliary neurotrophic factor in a rat sciatic nerve injury model. Arch Otolaryngol Head Neck Surg. 1998;124:761–766. doi: 10.1001/archotol.124.7.761. [DOI] [PubMed] [Google Scholar]
- Hoh JF. Laryngeal muscle fibre types. Acta Physiol Scand. 2005;183:133–149. doi: 10.1111/j.1365-201X.2004.01402.x. [DOI] [PubMed] [Google Scholar]
- Hoke A, Gordon T, Zochodne DW, Sulaiman OA. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp Neurol. 2002;173:77–85. doi: 10.1006/exnr.2001.7826. [DOI] [PubMed] [Google Scholar]
- Hudson TW, Evans GR, Schmidt CE. Engineering strategies for peripheral nerve repair. Clin Plast Surg. 1999;26:617–628. [PubMed] [Google Scholar]
- Ito Y, Yamamoto M, Li M, Doyu M, et al. Differential temporal expression of mRNAs for ciliary neurotrophic factor (CNTF), leukemia inhibitory factor (LIF), interleukin-6 (IL-6), and their receptors (CNTFR alpha, LIFR beta, IL-6R alpha and gp130) in injured peripheral nerves. Brain Res. 1998;793:321–327. doi: 10.1016/s0006-8993(98)00242-x. [DOI] [PubMed] [Google Scholar]
- Iwase T, Jung CG, Bae H, Zhang M, Soliven B. Glial cell line-derived neurotrophic factor-induced signaling in Schwann cells. J Neurochem. 2005;94:1488–1499. doi: 10.1111/j.1471-4159.2005.03290.x. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Hempstead BL, Martin-Zanca D, Chao MV, Parada LF. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991;252:554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Kaisho Y, Shintani A, Nagahama M, Kato K. Tissue distribution and immunocytochemical localization of neurotrophin-3 in the brain and peripheral tissues of rats. J Neurochem. 1996;66:330–337. doi: 10.1046/j.1471-4159.1996.66010330.x. [DOI] [PubMed] [Google Scholar]
- Kingham PJ, Birchall MA, Burt R, Jones A, Terenghi G. Reinnervation of laryngeal muscles: a study of changes in myosin heavy chain expression. Muscle Nerve. 2005a;32:761–766. doi: 10.1002/mus.20409. [DOI] [PubMed] [Google Scholar]
- Kingham PJ, Birchall MA, Terenghi G. Neurotrophin expression in laryngeal muscles following denervation and reinnervation. J Peri Nerv Sys. 2005b;10(Suppl.):41. [Google Scholar]
- Kirsch M, Terheggen U, Hofmann HD. Ciliary neurotrophic factor is an early lesion-induced retrograde signal for axotomized facial motoneurons. Mol Cell Neurosci. 2003;24:130–138. doi: 10.1016/s1044-7431(03)00130-1. [DOI] [PubMed] [Google Scholar]
- Kitahara AK, Suzuki Y, Qi P, et al. Facial nerve repair using a collagen conduit in cats. Scand J Plast Reconstr Surg Hand Surg. 1999;33:187–193. doi: 10.1080/02844319950159442. [DOI] [PubMed] [Google Scholar]
- Kopp DM, Trachtenberg JT, Thompson WJ. Glial growth factor rescues Schwann cells of mechanoreceptors from denervation-induced apoptosis. J Neurosci. 1997;17:6697–6706. doi: 10.1523/JNEUROSCI.17-17-06697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrador RO, Buti M, Navarro X. Influence of collagen and laminin gels concentration on nerve regeneration after resection and tube repair. Exp Neurol. 1998;149:243–252. doi: 10.1006/exnr.1997.6650. [DOI] [PubMed] [Google Scholar]
- Lee DA, Zurawel RH, Windebank AJ. Ciliary neurotrophic factor expression in Schwann cells is induced by axonal contact. J Neurochem. 1995;65:564–568. doi: 10.1046/j.1471-4159.1995.65020564.x. [DOI] [PubMed] [Google Scholar]
- Leibrock J, Lottspeich F, Hohn A, et al. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989;341:149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, Hamburger V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool. 1951;116:321–361. doi: 10.1002/jez.1401160206. [DOI] [PubMed] [Google Scholar]
- Lie DC, Weis J. GDNF expression is increased in denervated human skeletal muscle. Neurosci Lett. 1998;250:87–90. doi: 10.1016/s0304-3940(98)00434-0. [DOI] [PubMed] [Google Scholar]
- Lomo T, Westgaard RH, Dahl HA. Contractile properties of muscle: control by pattern of muscle activity in the rat. Proc R Soc Lond B Biol Sci. 1974;187:99–103. doi: 10.1098/rspb.1974.0064. [DOI] [PubMed] [Google Scholar]
- MacLennan AJ, Neitzel KL, Devlin BK, et al. In vivo localization and characterization of functional ciliary neurotrophic factor receptors which utilize JAK-STAT signaling. Neuroscience. 2000;99:761–772. doi: 10.1016/s0306-4522(00)90236-7. [DOI] [PubMed] [Google Scholar]
- Mahanthappa NK, Anton ES, Matthew WD. Glial growth factor 2, a soluble neuregulin, directly increases Schwann cell motility and indirectly promotes neurite outgrowth. J Neurosci. 1996;16:4673–4683. doi: 10.1523/JNEUROSCI.16-15-04673.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmgren LT, Jones CE, Bookman LM. Muscle fiber and satellite cell apoptosis in the aging human thyroarytenoid muscle: a stereological study with confocal laser scanning microscopy. Otolaryngol Head Neck Surg. 2001;125:34–39. doi: 10.1067/mhn.2001.116449. [DOI] [PubMed] [Google Scholar]
- Matheson CR, Carnahan J, Urich JL, Bocangel D, Zhang TJ, Yan Q. Glial cell line-derived neurotrophic factor (GDNF) is a neurotrophic factor for sensory neurons: comparison with the effects of the neurotrophins. J Neurobiol. 1997;32:22–32. [PubMed] [Google Scholar]
- McKay Hart A, Brannstrom T, Wiberg M, Terenghi G. Primary sensory neurons and satellite cells after peripheral axotomy in the adult rat: timecourse of cell death and elimination. Exp Brain Res. 2002;142:308–318. doi: 10.1007/s00221-001-0929-0. [DOI] [PubMed] [Google Scholar]
- Meyer M, Matsuoka I, Wetmore C, Olson L, Thoenen H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol. 1992;119:45–54. doi: 10.1083/jcb.119.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel RN, Parry DJ, Dunn SE. Regulation of myosin heavy chain expression in adult rat hindlimb muscles during short-term paralysis: comparison of denervation and tetrodotoxin-induced neural inactivation. FEBS Lett. 1996;391:39–44. doi: 10.1016/0014-5793(96)00618-7. [DOI] [PubMed] [Google Scholar]
- Midha R, Munro CA, Dalton PD, Tator CH, Shoichet MS. Growth factor enhancement of peripheral nerve regeneration through a novel synthetic hydrogel tube. J Neurosurg. 2003;99:555–565. doi: 10.3171/jns.2003.99.3.0555. [DOI] [PubMed] [Google Scholar]
- Minghetti L, Goodearl AD, Mistry K, Stroobant P. Glial growth factors I–III are specific mitogens for glial cells. J Neurosci Res. 1996;43:684–693. doi: 10.1002/(SICI)1097-4547(19960315)43:6<684::AID-JNR5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Mittal S, Cohen A, Maysinger D. In vitro effects of brain derived neurotrophic factor released from microspheres. Neuroreport. 1994;5:2577–2582. doi: 10.1097/00001756-199412000-00043. [DOI] [PubMed] [Google Scholar]
- Mohanna PN, Terenghi G, Wiberg M. Composite PHB-GGF conduit for long nerve gap repair: a long-term evaluation. Scand J Plast Reconstr Surg Hand Surg. 2005;39:129–137. doi: 10.1080/02844310510006295. [DOI] [PubMed] [Google Scholar]
- Mootoosamy RC, Dietrich S. Distinct regulatory cascades for head and trunk myogenesis. Development. 2002;129:573–583. doi: 10.1242/dev.129.3.573. [DOI] [PubMed] [Google Scholar]
- Mosahebi A, Fuller P, Wiberg M, Terenghi G. Effect of allogeneic Schwann cell transplantation on peripheral nerve regeneration. Exp Neurol. 2002;173:213–223. doi: 10.1006/exnr.2001.7846. [DOI] [PubMed] [Google Scholar]
- Mosahebi A, Woodward B, Wiberg M, Martin R, Terenghi G. Retroviral labeling of Schwann cells: in vitro characterization and in vivo transplantation to improve peripheral nerve regeneration. Glia. 2001;34:8–17. doi: 10.1002/glia.1035. [DOI] [PubMed] [Google Scholar]
- Mosahebi A, Wiberg M, Terenghi G. Addition of fibronectin to alginate matrix improves peripheral nerve regeneration in tissue-engineered conduits. Tissue Eng. 2003;9:209–218. doi: 10.1089/107632703764664684. [DOI] [PubMed] [Google Scholar]
- Motoyoshi K, Hyodo M, Yamagata T, Gyo K. Restoring vocal fold movement after transection and immediate suturing of the recurrent laryngeal nerve with local application of basic fibroblast growth factor: an experimental study in the rat. Laryngoscope. 2004;114:1247–1252. doi: 10.1097/00005537-200407000-00020. [DOI] [PubMed] [Google Scholar]
- Nagano M, Suzuki H. Quantitative analyses of expression of GDNF and neurotrophins during postnatal development in rat skeletal muscles. Neurosci Res. 2003;45:391–399. doi: 10.1016/s0168-0102(03)00010-5. [DOI] [PubMed] [Google Scholar]
- Nitta A, Ohmiya M, Jin-nouchi T, et al. Endogenous neurotrophin-3 is retrogradely transported in the rat sciatic nerve. Neuroscience. 1999;88:679–685. doi: 10.1016/s0306-4522(98)00469-2. [DOI] [PubMed] [Google Scholar]
- Noden DM. The embryonic origins of avian cephalic and cervical muscles and associated connective tissues. Am J Anat. 1983;168:257–276. doi: 10.1002/aja.1001680302. [DOI] [PubMed] [Google Scholar]
- Omura T, Sano M, Omura K, et al. Different expressions of BDNF, NT3, and NT4 in muscle and nerve after various types of peripheral nerve injuries. J Peripher Nerv Syst. 2005;10:293–300. doi: 10.1111/j.1085-9489.2005.10307.x. [DOI] [PubMed] [Google Scholar]
- Paniello RC, West SE, Lee P. Laryngeal reinnervation with the hypoglossal nerve. I. Physiology, histochemistry, electromyography, and retrograde labeling in a canine model. Ann Otol Rhinol Laryngol. 2001;110:532–542. doi: 10.1177/000348940111000607. [DOI] [PubMed] [Google Scholar]
- Parkinson DB, Langner K, Namini SS, Jessen KR, Mirsky R. beta-Neuregulin and autocrine mediated survival of Schwann cells requires activity of Ets family transcription factors. Mol Cell Neurosci. 2002;20:154–167. doi: 10.1006/mcne.2002.1109. [DOI] [PubMed] [Google Scholar]
- Pego AP, Poot AA, Grijpma DW, Feijen J. Copolymers of trimethylene carbonate and epsilon-caprolactone for porous nerve guides: synthesis and properties. J Biomater Sci Polym Ed. 2001;12:35–53. doi: 10.1163/156856201744434. [DOI] [PubMed] [Google Scholar]
- Perie S, St Guily JL, Callard P, Sebille A. Innervation of adult human laryngeal muscle fibers. J Neurol Sci. 1997;149:81–86. doi: 10.1016/s0022-510x(97)05395-1. [DOI] [PubMed] [Google Scholar]
- Perry VH, Brown MC, Gordon S. The macrophage response to central and peripheral nerve injury. A possible role for macrophages in regeneration. J Exp Med. 1987;165:1218–1223. doi: 10.1084/jem.165.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch RN. Low molecular weight complexed poly (3-hydroxybutyrate): a dynamic and versatile molecule in vivo. Can J Microbiol. 1995;41(Suppl. 1):50–54. doi: 10.1139/m95-167. [DOI] [PubMed] [Google Scholar]
- Rhee HS, Lucas CA, Hoh JF. Fiber types in rat laryngeal muscles and their transformations after denervation and reinnervation. J Histochem Cytochem. 2004;52:581–590. doi: 10.1177/002215540405200503. [DOI] [PubMed] [Google Scholar]
- Saika T, Senba E, Noguchi K, et al. Effects of nerve crush and transection on mRNA levels for nerve growth factor receptor in the rat facial motoneurons. Brain Res Mol Brain Res. 1991;9:157–160. doi: 10.1016/0169-328x(91)90142-k. [DOI] [PubMed] [Google Scholar]
- Saito K, Shiotani A, Watabe K, Moro K, Fukuda H, Ogawa K. Adenoviral GDNF gene transfer prevents motoneuron loss in the nucleus ambiguus. Brain Res. 2003;962:61–67. doi: 10.1016/s0006-8993(02)03933-1. [DOI] [PubMed] [Google Scholar]
- Sanuki T, Yumoto E, Komori M, Hyodo M. Expression of fibroblast growth factor-2 in the nucleus ambiguus following recurrent laryngeal nerve injury in the rat. Laryngoscope. 2000;110:2128–2134. doi: 10.1097/00005537-200012000-00030. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Seddon HJ. Three types of nerve injury. Brain. 1943;66:237–288. [Google Scholar]
- Sendtner M, Carroll P, Holtmann B, Hughes RA, Thoenen H. Ciliary neurotrophic factor. J Neurobiol. 1994;25:1436–1453. doi: 10.1002/neu.480251110. [DOI] [PubMed] [Google Scholar]
- Sendtner M, Holtmann B, Hughes RA. The response of motoneurons to neurotrophins. Neurochem Res. 1996;21:831–841. doi: 10.1007/BF02532307. [DOI] [PubMed] [Google Scholar]
- Shiotani A, Flint PW. Myosin heavy chain composition in rat laryngeal muscles after denervation. Laryngoscope. 1998;108:1225–1229. doi: 10.1097/00005537-199808000-00023. [DOI] [PubMed] [Google Scholar]
- Siegel SG, Patton B, English AW. Ciliary neurotrophic factor is required for motoneuron sprouting. Exp Neurol. 2000;166:205–212. doi: 10.1006/exnr.2000.7528. [DOI] [PubMed] [Google Scholar]
- Simon M, Terenghi G, Green CJ, Coulton GR. Differential effects of NT-3 on reinnervation of the fast extensor digitorum longus (EDL) and the slow soleus muscle of rat. Eur J Neurosci. 2000;12:863–871. doi: 10.1046/j.1460-9568.2000.00975.x. [DOI] [PubMed] [Google Scholar]
- Simon M, Mann D, Coulton G, Terenghi G. Differential tyrosine kinase C mRNA distribution in extensor digitorum longus and soleus motoneurons in adult rats: effect of axotomy and neurotrophin-3 treatment. Neurosci Lett. 2002;320:9–12. doi: 10.1016/s0304-3940(02)00007-1. [DOI] [PubMed] [Google Scholar]
- Simon M, Porter R, Brown R, Coulton GR, Terenghi G. Effect of NT-4 and BDNF delivery to damaged sciatic nerves on phenotypic recovery of fast and slow muscles fibres. Eur J Neurosci. 2003;18:2460–2466. doi: 10.1046/j.1460-9568.2003.02978.x. [DOI] [PubMed] [Google Scholar]
- Smith GM, Rabinovsky ED, McManaman JL, Shine HD. Temporal and spatial expression of ciliary neurotrophic factor after peripheral nerve injury. Exp Neurol. 1993;121:239–247. doi: 10.1006/exnr.1993.1091. [DOI] [PubMed] [Google Scholar]
- Sobue G, Yamamoto M, Doyu M, Li M, Yasuda T, Mitsuma T. Expression of mRNAs for neurotrophins (NGF, BDNF, and NT-3) and their receptors (p75NGFR, trk, trkB, and trkC) in human peripheral neuropathies. Neurochem Res. 1998;23:821–829. doi: 10.1023/a:1022434209787. [DOI] [PubMed] [Google Scholar]
- Squinto SP, Stitt TN, Aldrich TH, et al. trkB encodes a functional receptor for brain-derived neurotrophic factor and neurotrophin-3 but not nerve growth factor. Cell. 1991;65:885–893. doi: 10.1016/0092-8674(91)90395-F. [DOI] [PubMed] [Google Scholar]
- Sterne GD, Brown RA, Green CJ, Terenghi G. Neurotrophin-3 delivered locally via fibronectin mats enhances peripheral nerve regeneration. Eur J Neurosci. 1997a;9:1388–1396. doi: 10.1111/j.1460-9568.1997.tb01493.x. [DOI] [PubMed] [Google Scholar]
- Sterne GD, Coulton GR, Brown RA, Green CJ, Terenghi G. Neurotrophin-3-enhanced nerve regeneration selectively improves recovery of muscle fibers expressing myosin heavy chains 2b. J Cell Biol. 1997b;139:709–715. doi: 10.1083/jcb.139.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundback CA, Shyu JY, Wang Y, et al. Biocompatibility analysis of poly (glycerol sebacate) as a nerve guide material. Biomaterials. 2005;26:5454–5464. doi: 10.1016/j.biomaterials.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74:491–516. doi: 10.1093/brain/74.4.491. [DOI] [PubMed] [Google Scholar]
- Tang S, Shen YJ, DeBellard ME, et al. Myelin-associated glycoprotein interacts with neurons via a sialic acid binding site at ARG118 and a distinct neurite inhibition site. J Cell Biol. 1997;138:1355–1366. doi: 10.1083/jcb.138.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenghi G. Peripheral nerve regeneration and neurotrophic factors. J Anat. 1999;194:1–14. doi: 10.1046/j.1469-7580.1999.19410001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toews AD, Barrett C, Morell P. Monocyte chemoattractant protein 1 is responsible for macrophage recruitment following injury to sciatic nerve. J Neurosci Res. 1998;53:260–267. doi: 10.1002/(SICI)1097-4547(19980715)53:2<260::AID-JNR15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Tonra JR, Curtis R, Wong V, et al. Axotomy upregulates the anterograde transport and expression of brain-derived neurotrophic factor by sensory neurons. J Neurosci. 1998;18:4374–4383. doi: 10.1523/JNEUROSCI.18-11-04374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utley DS, Lewin SL, Cheng ET, Verity AN, Sierra D, Terris DJ. Brain-derived neurotrophic factor and collagen tubulization enhance functional recovery after peripheral nerve transection and repair. Arch Otolaryngol Head Neck Surg. 1996;122:407–413. doi: 10.1001/archotol.1996.01890160047009. [DOI] [PubMed] [Google Scholar]
- Viguie CA, Lu DX, Huang SK, Rengen H, Carlson BM. Quantitative study of the effects of long-term denervation on the extensor digitorum longus muscle of the rat. Anat Rec. 1997;248:346–354. doi: 10.1002/(SICI)1097-0185(199707)248:3<346::AID-AR7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Wang L, Copray S, Brouwer N, Meek MF, Kernell D. Regional distribution of slow-twitch muscle fibers after reinnervation in adult rat hindlimb muscles. Muscle Nerve. 2002;25:805–815. doi: 10.1002/mus.10114. [DOI] [PubMed] [Google Scholar]
- Wang MC, Forsberg NE. Effects of ciliary neurotrophic factor (CNTF) on protein turnover in cultured muscle cells. Cytokine. 2000;12:41–48. doi: 10.1006/cyto.1999.0516. [DOI] [PubMed] [Google Scholar]
- Wehrwein EA, Roskelley EM, Spitsbergen JM. GDNF is regulated in an activity-dependent manner in rat skeletal muscle. Muscle Nerve. 2002;26:206–211. doi: 10.1002/mus.10179. [DOI] [PubMed] [Google Scholar]
- Wells DG, McKechnie BA, Kelkar S, Fallon JR. Neurotrophins regulate agrin-induced postsynaptic differentiation. Proc Natl Acad Sci USA. 1999;96:1112–1117. doi: 10.1073/pnas.96.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler EF, Bothwell M. Spatiotemporal patterns of expression of NGF and the low-affinity NGF receptor in rat embryos suggest functional roles in tissue morphogenesis and myogenesis. J Neurosci. 1992;12:930–945. doi: 10.1523/JNEUROSCI.12-03-00930.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth IH, Brown RA, Dore C, Green CJ, Terenghi G. Orientated mats of fibronectin as a conduit material for use in peripheral nerve repair. J Hand Surg [Br] 1995;20:429–436. doi: 10.1016/s0266-7681(05)80148-2. [DOI] [PubMed] [Google Scholar]
- Wiberg M, Terenghi G. Will it be possible to produce peripheral nerves? Surg Technol Int. 2003;11:303–310. [PubMed] [Google Scholar]
- Wong V, Arriaga R, Ip NY, Lindsay RM. The neurotrophins BDNF, NT-3 and NT-4/5, but not NGF, up-regulate the cholinergic phenotype of developing motor neurons. Eur J Neurosci. 1993;5:466–474. doi: 10.1111/j.1460-9568.1993.tb00513.x. [DOI] [PubMed] [Google Scholar]
- Wu YZ, Crumley RL, Armstrong WB, Caiozzo VJ. New perspectives about human laryngeal muscle: single-fiber analyses and interspecies comparisons. Arch Otolaryngol Head Neck Surg. 2000;126:857–864. doi: 10.1001/archotol.126.7.857. [DOI] [PubMed] [Google Scholar]
- Yao M, Moir MS, Wang MZ, To MP, Terris DJ. Peripheral nerve regeneration in CNTF knockout mice. Laryngoscope. 1999;109:1263–1268. doi: 10.1097/00005537-199908000-00015. [DOI] [PubMed] [Google Scholar]
- Yoshii S, Oka M. Collagen filaments as a scaffold for nerve regeneration. J Biomed Mater Res. 2001;56:400–405. doi: 10.1002/1097-4636(20010905)56:3<400::aid-jbm1109>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Zealear DL, Billante CR, Courey MS, et al. Reanimation of the paralyzed human larynx with an implantable electrical stimulation device. Laryngoscope. 2003;113:1149–1156. doi: 10.1097/00005537-200307000-00010. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Luo XG, Xian CJ, Liu ZH, Zhou XF. Endogenous BDNF is required for myelination and regeneration of injured sciatic nerve in rodents. Eur J Neurosci. 2000;12:4171–4180. [PubMed] [Google Scholar]
- Zhang CG, Ma JJ, Terenghi G, Mantovani C, Wiberg M. Phrenic nerve transfer in the treatment of brachial plexus avulsion: an experimental study of nerve regeneration and muscle morphology in rats. Microsurgery. 2004;24:232–240. doi: 10.1002/micr.20015. [DOI] [PubMed] [Google Scholar]
- Zhou XF, Deng YS, Chie E, et al. Satellite-cell-derived nerve growth factor and neurotrophin-3 are involved in noradrenergic sprouting in the dorsal root ganglia following peripheral nerve injury in the rat. Eur J Neurosci. 1999;11:1711–1722. doi: 10.1046/j.1460-9568.1999.00589.x. [DOI] [PubMed] [Google Scholar]
- Zwick M, Teng L, Mu X, Springer JE, Davis BM. Overexpression of GDNF induces and maintains hyperinnervation of muscle fibers and multiple end-plate formation. Exp Neurol. 2001;171:342–350. doi: 10.1006/exnr.2001.7753. [DOI] [PubMed] [Google Scholar]