Figure 4. Crystal structures of Tgt(Glu235Gln).
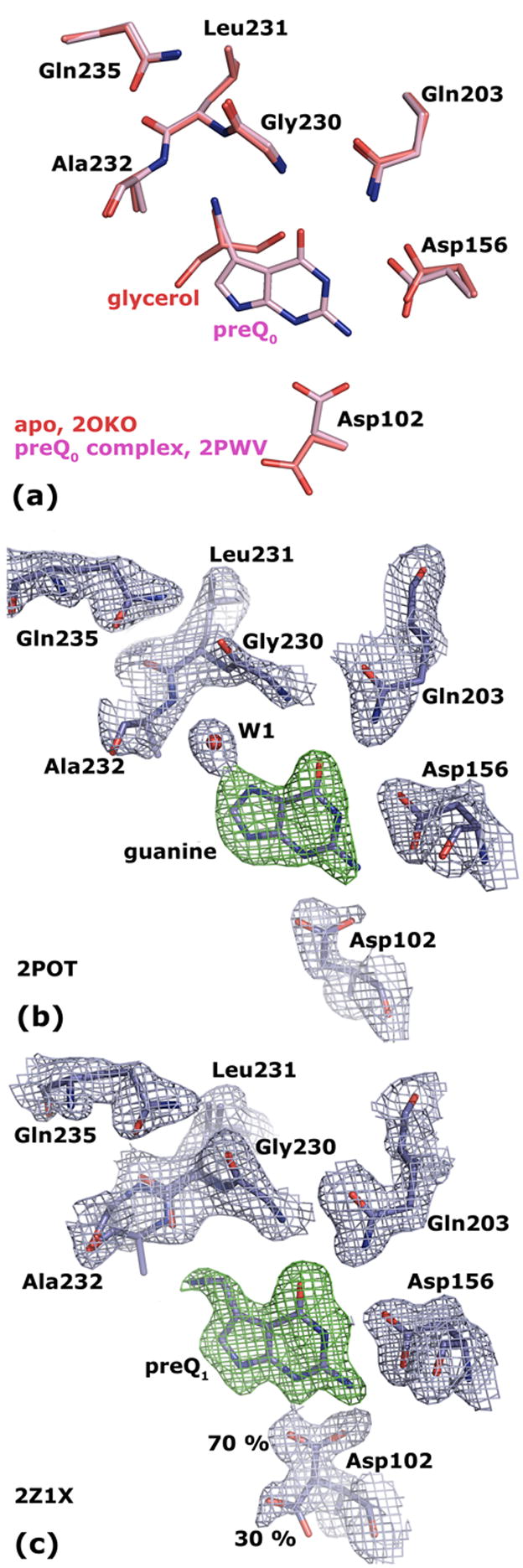
(a) Superposition of Tgt(Glu235Gln) in its apo form (crystallised at pH 5.5; the structure obtained at pH 8.5 looks identical) and in complex with preQ0 reveals equal binding pocket geometries. (b) 2 |Fo| − |Fc| electron density map of Tgt(Glu235Gln) in complex with guanine contoured at 1.5 σ (blue). The green density represents an |Fo| − |Fc| map with the ligand omitted from the calculation contoured at 3.0 σ. (c) 2 |Fo| − |Fc| electron density map of Tgt(Glu235Gln) in complex with preQ1 contoured at 1.5σ (blue). The green density represents an |Fo| − |Fc| map with the ligand omitted from the calculation contoured at 3.0 σ. The electron density reveals a split conformation for the Asp102 side chain indicating an uncomplete occupancy of the binding pocket with the substrate base. The Leu231/Ala232 peptide bond is present in two different conformations. The occupancy of the ligand and of the predominant Leu231/Ala232 and Asp102 conformers refine to 0.7.
