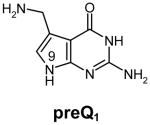
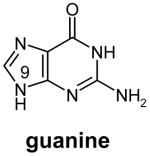
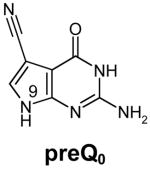
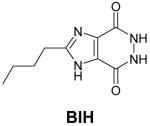
Table 1.
Structures of natural substrates of bacterial Tgt and the inhibitor 2-butyl-1H-imidazole-4,5-dicarboxylic acid hydrazide (BIH). In the line “conformation” the functional group of the Leu231/Ala232 peptide bond exposed to the binding pocket is given.