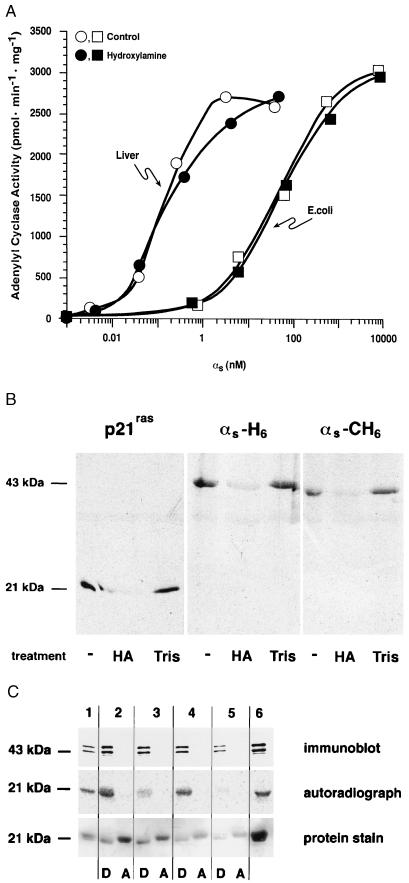
Figure 5.
Treatment of native and recombinant αs with hydroxylamine. (A) αs was purified from rabbit liver (○, •) or after expression in E. coli (□, ▪). Proteins were activated with GTP[γS] and then incubated with 1 M hydroxylamine at pH 7.0 (•, ▪) or 1 M Tris⋅HCl, pH 7.0 (○, □), for 30 min at room temperature. Proteins were gel filtered prior to reconstitution at the indicated concentrations with membranes (10 μg) from Sf9 cells expressing type V adenylyl cyclase and assay of αs-stimulated adenylyl cyclase activity. (B) His6-tagged (N terminus) p21ras, αs-H6, and αs-CH6 were synthesized in Sf9 cells and labeled in vivo with [3H]palmitic acid. Proteins were enriched by Ni2+-NTA chromatography and treated with 1 M hydroxylamine (HA) or 1 M Tris⋅HCl (Tris) as described for A. Samples were then subjected to sodium dodecylsulfate/polyacrylamide gel electrophoresis and fluorography. An untreated sample is shown in the first lane for each protein. (C) Purified rabbit liver αs was mixed in 20 mM Tris⋅HCl, pH 7.0/150 mM NaCl with p21ras that had been metabolically labeled with [3H]palmitic acid; this starting material is shown in lanes 1, 2, and 6. Aliquots of this mixture were treated with palmitoyl-protein thioesterase (lane 3), 1 M Tris⋅HCl, pH 7.0 (lane 4), or 1 M hydroxylamine, pH 7.0 (lane 5) and then subjected to Triton X-114 phase partitioning as described in the legend of Fig. 4. Proteins were resolved by electrophoresis, transferred to nitrocellulose, and detected by immunoblotting (with an αs-specific antibody), autoradiography, or protein staining (with Ponceau S).