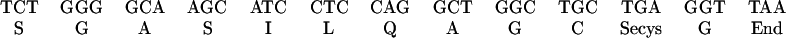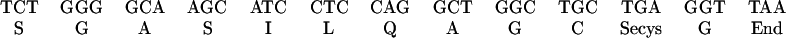Automated Edman degradation amino acid
sequences of tryptic selenopeptides derived from carboxymethylated
heparin high-affinity and heparin low-affinity forms of TR isolated
from human lung adenocarcinoma cells. The amino acid sequence of each
75Se-labeled tryptic peptide was determined using
Hewlett–Packard Protein Sequencer G100A. Eluates from each cycle were
collected and analyzed for
75Se using a gamma counter. The
amino acids identified in each cycle were identical for both peptides
and are indicated in the line below the figure. The profile of
radioactivity from the
75Se-labeled tryptic peptide
isolated from heparin high-affinity TR is shown in the bar graph of the
figure. The peak of radioactivity collected from the heparin
low-affinity
75Se-labeled peptide, likewise, was centered
in cycle 11 with the following profile: cycle 10, 245 cpm; cycle 11,
660 cpm; cycle 12, 317 cpm; cycle 13, 117 cpm. In this peptide, the
amino acid residue at cycle 11 was identified as dehydroalanine. In
both peptides, S-carboxymethylcysteine was identified in cycle 10. The
yield of the expected C-terminal glycine residue from both peptides was
too low to be detected. The gene sequence of the C-terminal region of
human placental TR (
11) is: