Abstract
At the time of Golgi and Cajal’s reception of the Nobel Prize in 1906 most scientists had accepted the notion that neurons are independent units. Although neuroscientists today still believe that neurons are independent anatomical units, functionally, it is thought that some sort of population coding occurs. Throughout this essay, we provide evidence that suggests that populations of neurons can code information through the synchronization of their responses. This synchronization occurs at several levels in the brain. Whereas spike synchrony refers to the correlation between spikes of different neurons’ spike trains, oscillatory synchrony refers to the synchronization of oscillatory responses, generally among large groups of neurons. In the first section of this essay we describe the dependence of the brain’s developmental processes on synchronous firing and how these processes form a brain that supports and is sensitive to synchronous firing. Data are then presented that suggest that spike and oscillatory synchrony may serve as useful neural codes. Examples from sensory (auditory, olfactory and somatosensory), motor and higher (attention, memory) systems are then presented to illustrate potential roles for these synchronous codes in normal brain function. Results from these studies collectively suggest that spike synchrony in sensory and motor systems may provide stimulus detail information not available from changes in firing rate. Oscillatory synchrony, on the other hand, may be globally involved in the coordination of long-distance neuronal communication during higher cognitive processes. These concepts represent a dramatic shift in direction since the times of Golgi and Cajal.
Keywords: Synchrony, Oscillations, Visual Cortex, Golgi, Coding
1. Introduction
The collected essays in this book grew out of a symposium celebrating the one hundred year anniversary of the awarding of the Nobel Prize to Camillo Golgi and Ramon y Cajal in 1906. Our task in these essays has been to examine how concepts of the neuron have changed in the past century. In keeping with that endeavor it is worth briefly turning back the clock to the time when the original award was made. The experimental advance made by Golgi’s discovery of the heavy metal impregnation technique, now known as the Golgi method, was the major technological advance that heralded modern anatomical analysis of the nervous system. The prize was awarded for Golgi and Cajal’s pioneering efforts using this technique to understand the structure of the nervous system. Relevant to the present chapter is the fact that Golgi and Cajal came to vastly different conclusions using the same method. While Cajal defended the idea of the neuron as an independent functional unit of the nervous system, Golgi clung to his belief that neurons were connected to one another in a reticular network. Golgi championed this idea in his Nobel address in spite of the fact that the majority of neuroanatomists in 1906 had already accepted the idea of the neuron as an independent functional unit. Golgi made the following remark in his Nobel address relevant to this issue, “I have never had reason, up to now, to give up the concept which I have always stressed, that nerve cells, instead of working individually, act together.” In spite of the fact that Golgi’s ideas were dismissed as outmoded, his statement about neural function in his Nobel address has a surprisingly modern ring. Although Golgi was not as concerned about function as about structure, his statement captures the essence of what we know to be true and that is that neurons must functionally act together. So how is this accomplished?
Golgi and Cajal were not physiologists, but both were aware of relevant publications in the field at the time that reflected thinking about function in the nervous system. Both Golgi and Cajal, but especially Cajal, were interested in how anatomy might translate into function. Along those lines, it is noteworthy that the very same year that Golgi and Cajal were awarded the Nobel Prize, Sir Charles Sherrington published his famous book entitled The Integrative Action of the Nervous System, which describes some of his seminal work on spinal cord reflexes among other observations (Sherrrington, 1906). Cajal and others were strongly influenced by Sherrington’s physiological studies of reflexes and by the concept of a “synapse,” first proposed by Sherrington. Sherrington, who would win the Nobel Prize himself 26 years later in 1932, also was strongly influenced by the anatomical descriptions of both Golgi and Cajal. Of course, Sherrington supported the neuron doctrine as espoused by Cajal. As a physiologist, however, he also emphasized the coordination of networks of neurons and described clearly the coordinated walking pattern that could be elicited from spinal cats (cats whose cervical spinal cords were sectioned), which he attributed to the precise timing of local communication within the spinal cord, between neurons and between neurons and muscles. In his later years, Sherrington (1941), in fact, hypothesized that communication between neurons likely involved some sort of a “population” code that might contain emergent properties not available in the collective responses of single neurons. Additionally, Lord Adrian (1942) more then 50 years ago showed clearly that populations of olfactory bulb neurons showed coordinated activity when presented with particular odorants which he described as “induced waves”. So, in Golgi’s words, nerve cells “act together”.
Returning to the purpose of the book, namely to explore how the concept of the neuron has changed in the century since Golgi and Cajal presented their Nobel addresses, we argue here that while Golgi and Cajal used the Golgi method to reveal and debate the anatomical structure of neurons, a century later new sets of tools are available that address similar issues at a functional level. How does a population of neurons in Golgi’s words “act together” to code information? In this chapter we review evidence that an important way that local populations of neurons code information is through the synchrony of their action potentials. According to this view, the individual neuron is still relevant but its relevance functionally depends dynamically on which of several networks it belongs to in time. Information emerges from a cooperative local network although such local neurons and networks are also constrained by their individual anatomical connectivity.
Currently few investigators doubt the existence of some form of population coding given the broad tuning of individual neurons (Casagrande et al. 2002). For population coding to reliably occur, however, there has to be a consistent mechanism available for increasing the probability of transmitting a neural code among individual neurons within an assembly or network, while (at the same time) distinguishing those neurons that belong to the assembly from those neurons that do not. The most commonly suggested mechanism for increasing response transmission is neuronal synchrony, temporal correlations of neuronal responses on the scale of milliseconds. There are different degrees of neuronal synchrony, which differ in the number of neurons whose responses are synchronized (for review see Singer, 1993; Singer and Gray, 1995; Usrey and Reid, 1999; Engel and Singer, 2001). Epilepsy is a global form of synchrony where many neurons in the brain may be synchronized to the detriment of function (for review see Vingerhoets, 2006). On a smaller scale, there are oscillations, where large networks of neurons synchronize with each other. Oscillatory synchrony has been implicated in several cognitive functions, including feature binding and scene segmentation (Singer and Gray, 1995), memory formation and recall (Tallon-Baudry and Bertrand, 1999) and attention (Fries et al., 2001). On the smallest scale there is spike synchrony, the temporal correlation of spikes belonging to a group of neurons within a local circuit or network (Usrey and Reid, 1999; Biederlack et al., 2006).
Synchrony is an attractive neuronal population coding mechanism because it is dynamic and therefore one neuron can belong to different network assemblies at different times. Additionally, synchronized spike timing increases the probability and speed of generating an action potential in a downstream neuron which must integrate signals from many inputs, especially in large brains (for review see Singer, 1993; Singer and Gray, 1995; Usrey and Reid, 1999; Engel and Singer, 2001). These characteristics are advantageous if synchrony between neurons is to code useful information.
Despite the attractiveness of neuronal synchrony as a mechanism for population coding, its acceptance remains controversial given alternative proposals that support only a rate code based on the magnitude of firing of individual neurons (Shadlen and Movshon, 1999). So what support is there for a code based on synchrony? In the first part of this review we consider how developmental mechanisms set the stage for coding via synchrony, given that co-activation of neurons promotes synaptogenesis. In this section we also show how the anatomical architecture which results is designed to support synchrony in the local communication among neurons and synchrony in the broader form of oscillations. We then examine the question “Can neuronal synchrony code useful information?” We argue that the answer is yes and that synchrony’s relevance can be demonstrated via examples from each of the major sensory systems with special emphasis on the visual system since the visual system has been studied in the greatest detail. We then examine the role of synchrony in higher cognitive processes. The idea that synchrony within networks might form the basis of memory has a long history which we summarize in this section. Additionally, we examine data that supports the idea that synchrony plays a role in attention, another higher cognitive process. Throughout this essay we review and distinguish between oscillatory and spike synchrony and argue that both types of correlated firing are important components of neural coding at several different levels in the brain.
2. Development, Anatomy and Synchrony
2.1. Synchrony promotes correct wiring
Mammalian development is a remarkable process in which one cell becomes a complete organism with many interconnected organ systems. The brain’s 100 billion neurons are further divided into many separate functional units with specific and distributed connections between them. These precise connections are shaped by many factors, such as molecular gradients, timing of axon arrival and correlated spontaneous activity. The earlier stages of brain patterning, where broad regions are defined and early connections are formed, does not require neural activity. It is, however, required for later stages of development, where axons, dendrites and synapses are precisely sculpted. Synchronous firing is especially important for normal nervous system development at this later stage. Numerous studies have provided evidence to support the idea that neurons that are active together during development tend to wire together and that synapses are either strengthened or lost based on the synchrony of their firing (Voronin et al., 1996; Kleschevnikov et al., 1997; Volgushev et al., 1997).
The idea that cells that fire together wire together is a derivative of Donald Hebb’s (1949) learning model. Hebb proposed that those pathways that are active together strengthen connections at the expense of those pathways that are not co-active. At the cellular level the proposed mechanism by which this process occurs involves the N-methyl-D-aspartate (NMDA) class of glutamate receptors which are particularly prominent in many areas of the brain during development. Binding of glutamate at the NMDA receptor coupled with a strong depolarization removes the channel’s magnesium block allowing the influx of calcium through the channel. Calcium activates intracellular kinases that lead to pre and postsynaptic changes that either allow synapses to form or strengthen existing synapses. NMDA antagonists have been shown to disrupt the normal process of synaptogenesis during development (Vislay-Meltzer et al. 2006; see for review Constantine-Paton and Cline, 1998 and Casagrande and Wiencken-Barger, 2000).
The dependence of neural development on correlated firing has been most heavily documented in the visual system. It has been argued that correlated firing among retinal neurons occurs even in utero, before exposure to visual stimuli, in the form of waves of spontaneous synchronous activity (temporally distinct for each eye and each retinal region) and that these waves can help pattern synaptogenesis of retinal ganglion cell axons in their thalamic target, the lateral geniculate nucleus (LGN) (Stellwagen and Shatz, 2002). During the stage that retinal ganglion cell axons are growing, retinal, thalamic and cortical neurons are heavily interconnected by developmentally transient gap junctions. Gap junctions between neurons in a network are an effective way of inducing correlated firing, as knock-out of gap junction proteins has been shown to eliminate correlated firing among cells (Christie et al., 2005). The waves of correlated firing among retinal ganglion cells are important for the normal formation of separate ocular dominance regions in both the LGN and V1. This was demonstrated clearly in the now famous experiments of Carla Shatz’s (1988) group where it was shown that blocking all activity in fetal kittens in utero blocks the segregation of axons into ocular dominance territories in the LGN. Whether this same activity-dependent mechanism applies to the patterning of retinogeniculate axons across species is a matter of debate (See Chalupa this volume). Regardless, numerous experiments have shown that manipulation of retinal activity in early development can perturb normal development of cortical ocular dominance columns at a stage when geniculocortical axons are still very immature (for review see Casagrande and Wiencken-Barger, 2000).
2.2. Adult architecture promotes synchrony
Not surprisingly, based on the developmental rules just discussed, maps of feedforward, feedback and lateral connections in the brain are fairly precise with regions that represent common points in the periphery or common functional features being connected to one another (Lowel and Singer, 1992; Angelucci et al., 2002). The tendency for this architecture to support synchronous activity is best demonstrated by the waves of activity seen in EEG measurements and also in cases where synchrony becomes deleterious as is the case in epilepsy (for review see Vingerhoets, 2006). Additionally there are examples where cortical architecture appears to support sequences of synchronous activity in the form of “temporal modules” (Ikegaya et al. 2004). Feedback to thalamus from cortex also has been shown to play a role in the generation of synchrony. For example, Silito et al. (1994) demonstrated that cortical feedback between the visual cortex and LGN can induce correlation of responses in LGN cells whose receptive fields are appropriately aligned to signal the orientation of stimulus contour in the matching feedforward pathway. They hypothesized that this increases the gain of stimulus feature-specific input to the cortex through synchronizing the responses of thalamic neurons.
Within the cortex anatomical networks of GABAergic interneurons also have been shown to display extensive synchronous and oscillatory firing. Interneurons form networks through linking via both electrical and chemical synapses. Many different classes of interneurons with distinct and consistent firing patterns have been identified. In some areas these different networks of interneurons can display either synchronous (0 ms phase lag) or anti-synchronous (phase lag half of interspike interval) firing, which are state-dependent (Tamas et al., 2000; Nomura et al., 2003; Merriam et al., 2005). Although the functional relevance of these two states of interneuron activity is not understood, it is known that application of GABA receptor antagonists can disrupt brain synchrony (Stopfer et al., 1997; Macleod et al., 1998; Nusser et al., 2001).
Glial architecture also appears to support temporal cooperation in brain activity. Fellin et al. (2004) have elegantly shown a link between astrocytes and neural synchrony. They isolated slow inward currents in CA1 neurons of the hippocampus after stimulation of Schaffer collaterals. These currents persisted even when neuronal activity and synaptic glutamate release were blocked. They found that these currents are caused by calcium oscillations in astrocytes. The oscillating astrocytes release glutamate, which binds to extracellular NMDA receptors on CA1 neurons, causing synchronized slow inward currents. It should be remembered that astrocytes can link all regions of the brain and also link brain activity to metabolism given that all astrocytes are linked together via gap junctions between their endfeet that line the brain and all blood vessels and show special relationships to synaptic zones (Wiencken and Casagrande, 1999). These glial cells are thus in a position to modulate correlated firing throughout the brain.
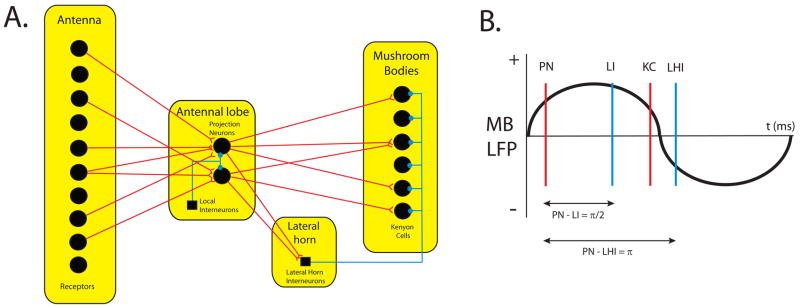
Several anatomical features appear well-suited to promote oscillatory activity. For example, pyramidal neurons in layer 5 of the visual cortex have been shown to have intrinsic oscillatory activity in the gamma frequency range (Gray and McCormick, 1996). Recurrent inhibition is another strong generator of oscillatory activity. The insect olfactory system has proven to be a great model for studying the role of recurrent inhibition as well as other mechanisms in generating oscillatory synchrony (See Fig. 1). Olfactory receptors project to principal neurons in the insect antennal lobe. The antennal lobe also contains local interneurons, which have been implicated in the generation of oscillations in the principal neurons. The local interneurons are interconnected via gap junctions. This interneuron population typically fires a quarter of an oscillatory cycle after principal neuron activation (Fig. 1B). This period corresponds to the period when the principal neurons are hyperpolarized. These inhibitory inputs further delay subsequent principal neuron firing, and because these inhibitory inputs are distributed and synchronized, the principal neurons’ action potentials also synchronize (20–35 Hz). The principal neurons project their oscillatory spikes to Kenyon cells in the insect mushroom body for more complex analysis. Collaterals from the principal neurons project to a small group of GABAergic neurons in the lateral horn, which in turn project to the Kenyon cells (Fig. 1A). The inhibitory neurons in the lateral horn receive oscillatory input and send oscillatory spikes to the Kenyon cells that lag the arrival of the principal neuron spikes by 173° (Fig. 1B). The Kenyon cells thus receive an alternation of oscillatory stimulatory input from the principal neurons and of oscillatory inhibitory input from the lateral horn neurons. The two groups of inhibitory neurons thus serve to improve the precision of stimulus-dependent oscillations in the insect olfactory system (Laurent et al., 1996; Stopfer et al., 1997; MacLeod et al., 1998; Stopfer and Laurent, 1999). This form of synchrony has been shown to be very important in providing olfactory information to higher areas in the insect brain, and as will be described later, disruption of this synchrony impairs olfactory discrimination in the insect (Stopfer et al., 1997). Similar mechanisms likely exist in mammals.
Figure 1.
Anatomy of the insect olfactory system promotes oscillatory synchrony. (A) Anatomy of the insect olfactory system. 90,000 olfactory receptors in the antenna project to 830 projection neurons in the antennal lobe. The projection neurons are heavily interconnected by about 300 local interneurons also in the antennal lobe. The projection neurons project to 50,000 Kenyon cells in the mushroom body. The projection neurons also send collaterals to 80 lateral horn interneurons in the insect lateral horn, which in turn, also send projections to the Kenyon cells. (B) Relationship of each cell type’s spike timing relative to a mushroom body local field potential (LFP). The recurrent inhibition of the insect olfactory system generates very precise oscillatory spikes in each cell type. Local interneuron spikes in the antennal lobe lag projection neuron spikes by one quarter of an oscillatory cycle, prolonging projection neuron hyperpolarization. Inhibitory lateral horn interneuron spikes onto Kenyon cells lag the stimulatory projection neuron spikes onto Kenyon cells by half an oscillatory cycle. Both these mechanisms serve to increase the precision of oscillatory spike synchrony. See text for details.
2.3. Oscillatory synchrony vs. spike synchrony
Although it is clear that wiring designed to support oscillatory synchrony would also support local spike synchrony, the relationship between the two has proven difficult to elucidate (see also Singer, this volume). Gray et al. (1989a,b) demonstrated that synchronized cells separated by large cortical distances generally oscillate in the gamma frequency range (30–50 Hz). It was later proposed that these oscillations are a mechanism for generating long-range synchrony (Singer, 1993; Eckhorn, 1994, Engel and Singer, 2001). This idea has received support from several simulation studies (Schuster and Wagner, 1990a,b).
Although spike synchrony can occur without oscillatory patterning, oscillatory synchrony always generates spike synchrony. This occurs because an oscillation is an alternation between states of inhibition and increased excitability. When a population is oscillating, its neurons will generally fire during the latter state, resulting in an increase in population synchrony. The greater the oscillation amplitude, the greater the precision of the synchrony. Oscillations have, for this reason, been hypothesized to be a mechanism of increasing spike timing precision of neuronal assemblies (for review see Singer, 1993; Singer and Gray, 1995; Usrey and Reid, 1999; Engel and Singer, 2001; Samonds and Bonds, 2005). In addition, experimental evidence shows that response latencies may shift in a manner dependent on the polarity of the preceding local field potential (LFP) fluctuation. This suggests that temporally dispersed excitatory post-synaptic potentials (EPSPs) may become synchronized within less than an oscillatory cycle through the shifting of spike latencies (Crick and Koch, 1990; Fries et al., 2001).
Oscillations also have been considered to be an epiphenomenon of spike synchrony, occurring as a result of synchronous stimulus onset-dependent bursting activity (Singer, 1993). Oscillatory activity, however, has been found to be only weakly correlated with stimulus onset bursting activity (Samonds and Bonds, 2005). Oscillatory activity is more likely the result of internal network dynamics reflecting complex interactions between local networks that themselves may or may not be synchronized (Eckhorn, 2000).
Several lines of evidence, however, do not support a causal relationship between oscillatory synchrony and spike synchrony. Most studies indicate that oscillatory neurons are the small minority in the visual cortex, with several groups reporting the prevalence to be as low as 2–5% (Desimone and Schein, 1987, Eckhorn et al., 1988; Alais, 1998). In addition, a recent study (Samonds and Bonds, 2005) suggests that oscillations are not required for the generation of spike synchrony in cat visual cortex. By determining spike synchrony using a joint-peristimulus time histogram (Aertsen, 1989) and correlating this value with the degree of oscillatory activity of the cells as determined using autocorrelation, these authors showed that the presence of oscillations is only weakly correlated with the strength of spike synchrony. The latter study also showed that correlated activity is maintained longer in the presence of oscillatory activity, suggesting that although oscillations are not involved in the generation of local synchrony, they may be involved in its maintenance (Samonds and Bonds, 2005). A large limitation of all of these studies that describe the low incidence of oscillatory activity is that these studies examined oscillations by looking at single cell responses. Studies of spike field coherence, which relate neuron spiking activity to population oscillations, suggest that the majority of visual cortex neurons are oscillatory (Jarvis and Mitra, 2001; Womelsdorf et al., 2006). Much more research needs to be done to relate spike synchrony to oscillations. An obvious advantage of having these two types of correlations is that, as described above, spike synchrony is highly dependent on oscillations. Thus, spike synchrony generated in early visual areas may be subject to oscillatory patterning from higher brain areas.
Determining the source of oscillations and spike synchrony and their relationship has been confounded by the fact that neurons are highly nonlinear devices (for review see Crick and Koch, 2003). When such nonlinear devices are interconnected they produce emergent states of activity patterns with complex dynamics, which in several cases have been reproduced in simulation studies (Grossberg et al., 1989; Ermentrout et al., 1998; Ermentrout and Chow, 2002). It has been shown that very simple inter-neuronal connection patterns that are ubiquitous in many sub-cortical structures and cortical areas can cause the interconnected dynamical nonlinear devices to go into both stable and unstable periodic states, which can produce oscillations and synchrony (Grossberg, 2001; Ermentrout et al., 1998). Thus, although it is clear that brain architecture is conductive to promoting synchrony, either globally through oscillations or locally through spike synchrony, determining the sources of these types of activity and their relationship will involve not only further elucidation of cortical and sub-cortical microcircuitry, but also an understanding of how the components of these circuits cooperatively function in large populations given their nonlinear nature.
Throughout the remainder of this essay, where it is possible, we will continue to try to distinguish between oscillatory and spike synchrony as we think that these two complementary forms of synchrony may have different roles in neural coding. What remains to be determined, however, is whether the synchrony means anything functionally. It could be argued that both spike synchrony and oscillatory synchrony are just epiphenomena of developmental mechanisms. The next section will describe research that suggests that neural network codes based on synchrony can be effective in coding information above that produced simply by the magnitude of firing in individual neurons.
3. Can neural synchrony code useful information?
Before considering the functional relevance of neural synchrony, it needs to be shown that synchrony can code information that is ultimately useful to behavior. Without getting deeply into issues of coding and information theory, suffice it to say that the answer is complicated by the likely fact that the nervous system makes use of different coding strategies depending on the circumstances. It is clear that firing rate is correlated with many aspects of neural processing. What we are asking here is whether synchronous firing among populations of neurons provides more information or additional information than can be provided by the rate of firing of individual neurons. The answer also depends, in turn, on what time scales one is considering. The reader should be aware that definitions and forms of analysis vary significantly between investigators in the many studies that consider temporal coding in the nervous system. With that cautionary note in mind, we believe the key relevant questions that need to be answered concerning synchrony are: 1) Are spike trains temporally precise? 2) Does spike timing encode information not available from firing rate? 3) Can neurons operate as coincidence detectors? and 4) Can spike synchrony or oscillations among groups of neurons be transmitted from one level to the next? (for further review of these issues see Singer, 1993; Singer and Gray, 1995; Usrey and Reid, 1999; Engel and Singer, 2001). To answer these questions in this section, we primarily use examples from the visual system, since neuronal synchrony has been most extensively studied in this sensory system.
3.1. Are spike trains temporally precise?
Because synchrony utilizes the correlation of spikes on a millisecond timescale, to be a reproducible neural code, the spike trains of neurons should be temporally precise on a similar time scale. It has been suggested that large spike timing variability to repeated stimuli would indicate that only an integration mechanism based on firing rate could extract information from the spike train (Shadlen and Movshon, 1999). Although high temporal precision of neuronal spike trains is important for the generation of neuronal synchrony, it is important to distinguish between the precision of the spike train generated by a single neuron and the precision of the relatively co-occurring spikes in an ensemble of neurons. Even if the spike train of a single neuron is variable, when all the neurons in an assembly fire together (in response to a stimulus), they can fire their spikes in a coordinated manner that results in precise temporal coincidence that is sufficient to consistently elicit a downstream response. This was demonstrated in the rat barrel system by Bruno and Sakmann (2006). Using extracellular electrodes in the thalamus and intracellular electrodes in the cortex, it was shown that thalamocortical synapses are weak, but the inputs are highly convergent and synchronous, giving them the ability to strongly drive cortical neurons. The cortical neurons responded highly precisely to stimuli, although each individual thalamocortical projection provided only a small fraction of the stimulation. Thus although individual thalamic neurons could have trial to trial variation in spike timing, cortical neuron responses are highly correlated to the synchronous spikes of thalamic assemblies, which are highly precise. The Bruno and Sakmann (2006) study also had important implications for Zohary et al.’s (1994) study which revealed that the correlation among neuronal spike trains can be disadvantageous by propagating noise. Although correlations can reduce the benefits of averaging many inputs, when the activity of a population is integrated over time, its activity gets averaged and noise is reduced. Thus downstream responses can be very consistent even in light of noisy synchronous spikes among populations of neurons (Bruno and Sakmann, 2006).
Several studies suggest that neurons are precise in a manner that would allow for a code based on spike synchrony. High temporal precision was described early in the study of the auditory system, where differences in spike timing are generated by the relative distance from each ear to an auditory stimulus. The spikes generated by these neurons in the early auditory pathways are highly precise and the difference in timing between spikes generated by the separate ears is used to spatially localize the source of the sound (Oertel, 1999). Since interaural timing differences are an essential clue to localization in auditory space, however, it could be argued that they represent a special case and do not generalize to the nervous system as a whole. In the visual system, high temporal precision has also been demonstrated in the spike trains of retinal ganglion cells (Berry et al., 1997; Berry et al., 1998; Reich et al., 1997), LGN neurons (Berry et al., 1998; Reinagel and Reid, 2000; Kara et al., 2000), primary visual cortical (V1) neurons (Samonds et al., 2003; Samonds and Bonds, 2004; Kohn and Smith, 2005) and higher order visual cortical neurons in the middle temporal (MT) (Bair and Koch, 1994, 1996; Buracas et al., 1998) and inferior temporal (IT) (Richmond et al., 1987a,b; Optican and Richmond, 1987) visual areas of the primate. A general finding of these studies is that although trial to trial neuronal firing rates may be variable, the spikes that are produced are temporally precise to within 3–7 ms (less than the average interspike interval (ISI)) in a stimulus-dependent manner.
3.2. Does spike timing encode information not available from firing rate?
Several studies suggest that more visual information is encoded in the temporal properties of spikes than in firing rate alone. Information-theoretic analyses of spikes produced by retinal ganglion cells (Berry et al., 1997; Berry et al., 1998), LGN (Dan et al., 1998), V1 (Samonds et al., 2003; Samonds and Bonds, 2004; Samonds et al., 2004) and area MT neurons (Buracas et al., 1998) indicate that precise cooperative activity between pairs of neurons may account for 40 to more than 200% more information than independent firing depending upon the stimulus conditions. Importantly, it was shown that the amount of information gain was dependent on the degree of correlation between the pairs of neurons. Moreover, it also has been shown that the classically defined visual receptive fields encoded by the synchronous spikes of two retinal ganglion cells (Meister et al., 1995) or two cells in cat V1 (Ghose et al., 1994) are smaller than the receptive fields encoded by changes in firing rate of either neuron alone, suggesting that the spatio-temporal resolution of coincident spikes is greater than that of single units.
3.3. Can neurons operate as coincidence detectors?
Many studies have sought to determine whether visual neurons act as temporal integrators or coincidence detectors. For a neuron to be a temporal integrator, which is ideal for detection of changes in firing rate of the input neurons, the postsynaptic membrane integration time constant has to be greater than the average ISI of its inputs. On the other hand, short membrane integration time compared to the ISI is required for a neuron to be a coincidence detector, a mechanism well-suited for the discrimination of synchronous from asynchronous spikes (for review see König. et al., 1996; Usrey and Reid, 1999). Several modeling studies have indicated that neurons are highly sensitive to the synchronicity of input spikes (Softky, 1994; Lumer et al., 1997). These studies show that spikes synchronous to within four milliseconds significantly affect the firing rates and selectivity of neuronal responses to visual stimuli. The physiological determination of both the average ISI and the integration time constant has proven to be difficult, however, mainly due to the dependence of these values on the overall baseline level of activity. Early evidence indicated that the integration time constant is between 8–15 ms (for review see Abeles, 1982; Pei et al., 1994). This would be adequate for coincidence detection if neuronal firing rates consistently weren’t much higher than 25 Hz. Visual neurons with firing rates above 100 Hz, however, are commonly found. Nevertheless, theoretical data suggests that the integration time constant at small dendritic branches may be an order of magnitude smaller than at the soma (Agmon-Snir and Segev, 1993). In addition, some recent studies provide evidence showing that neurons are more sensitive to synchronous spikes under states of high activity. Using intracellular recordings and numerical simulations Azouz and Gray (2003) found dynamic changes in V1 neuron thresholds that increase the neuron’s sensitivity to correlated inputs and decrease it’s sensitivity to uncorrelated inputs. Taken together with simulation data that suggests that the postsynaptic potential (PSP) time constant is significantly shorter in highly active neurons (Bernander et al., 1991; Softky, 1994; Creutzfeldt, 1995), these data suggest that coincident input is required to generate action potentials, particularly in states of high activity. Chaos, the constant but variable activation of excitatory and inhibitory connections by broadly-tuned spikes, has also been shown to promote synchronous activity (Hansel, 1996; van Vreeswijk and Sompolinsky, 1996; Karbowski and Kopell, 2000). It has been suggested that chaotic activity maintains the postsynaptic potential near but just below threshold, reducing the integration time constant (Koch, 1996; Samonds et al., 2003), allowing neurons to be more sensitive to coincident inputs.
There also are a few physiological studies that indicate that synchronous spikes are better at generating responses than are asynchronous spikes. Electrophysiological data shows that synchronous spikes in the LGN (Singer, 1973; Singer and Bedworth, 1973) and primary visual cortex (Alonso et al., 1996; Usrey et al., 2000) sum supralinearly and are better at evoking action potentials than asynchronous spikes. In addition, recent evidence from our lab demonstrates that the degree of synchrony among neurons within a visual area correlates with how effective those neurons’ spikes are at generating downstream action potentials (Jermakowicz et al., 2006, 2007). Cross correlation histograms were computed between spike trains of neurons in areas V1, the secondary visual area (V2) and the tertiary visual area (V3) of the prosimian primate bush baby (galago). The timing of the peaks in the cross correlation histograms between neurons in the three different visual areas with overlapping receptive fields suggested that these represented connected sets of neurons with V1 driving activity in V2, and V2 driving activity in V3. Changing stimulus conditions, such as orientation, spatial and temporal frequency, changed the firing rates and synchrony among V1 neurons. The efficacy at which V1 neurons evoked spikes in areas V2 and V3 (as determined by peak height in the respective cross correlation histograms) was highly correlated with the amount of synchrony among V1 neurons, but not with the changes in firing rate (Jermakowicz et al., 2006, 2007). These data further demonstrate that synchronous spikes are better at evoking downstream spikes than asynchronous spikes during visual stimulation.
3.4. Are spike synchrony and oscillations transmitted between different levels?
The temporal precision of neuronal spike trains is maintained from the retina to extrastriate areas (Bair and Koch, 1996; Berry et al., 1997; Reich et al., 1997; Buracas et al., 1998; Berry et al., 1998; Reinagel et al., 1998; Reinagel and Reid, 2000, Samonds et al., 2003; Samonds and Bonds, 2004; Jermakowicz 2006, 2007), indicating that the visual system may be capable of reliably transmitting synchronous spikes. This is supported by the Silito et al. (1994) study described earlier that showed that feedback from V1 induces correlated firing in the cat LGN. The most convincing studies showing the serial transmission of correlated firing, however, were done by Neuenschwander et al. (1996) and Castelo-Branco et al. (1998). These studies reported that transient 60–120 Hz oscillations resulting from static stimuli may be transmitted from retina to V1. In contrast, moving stimuli produced cortically-generated 30–60 Hz oscillations and corticogeniculate feedback phase-locked LGN oscillations to this frequency.
Studies from our lab also suggest that synchronous signals may be transmitted through the early visual cortex. As mentioned previously, the amount of synchrony among neurons in a lower visual area determines how well spikes from those neurons evoke action potentials in higher order areas. These higher area neurons synchronize with each other, and their degree of synchrony is directly correlated with the amount of synchrony among the neurons driving them from the lower area. It is possible that the synchrony in both lower and higher areas may arise from separate sources and the synchrony in the higher area may not be a result of the synchrony in the lower area. However, the fact that spikes from the synchronous cells in the higher visual areas (V2, V3) precisely lag the spikes of the synchronous cells in the lower visual areas (V1, V2) suggests that synchronous signals are transmitted serially through the several levels of processing in the visual system. Our results also suggest that potential object-encoding synchronous assemblies become progressively sparser but more correlated as they travel through the early visual system (Jermakowicz et al, 2007). Sparse coding means that there is a low level of redundancy among neurons in what is coded, which is important given the energy demands of the nervous system. Sparse codes based on synchrony have also been observed in other brain systems (Perez-Orive et al., 2002; Hahnloser et al., 2002).
The data from the preceding sections collectively demonstrate that spike synchrony could serve as a reasonable and efficient neural code that is reliable, can be detected by post-synaptic neurons and can be transmitted from lower to higher levels in the visual hierarchy. The advantage of such a coding mechanism compared to one based solely on firing rates is that: 1) It is more rapid since coincidence detection is faster than the temporal integration that would be required for coding by firing rate (for review see König et al., 1996), 2) It is more dynamic and flexible (for review see Singer, 1993; Singer and Gray, 1995; Usrey and Reid, 1999; Engel and Singer, 2001), and 3) It can encode more information (Ghose et al., 1994; Meister et al., 1995; Berry et al., 1997; Berry et al., 1998; Buracas et al., 1998; Dan et al., 1998; Samonds et al., 2003; Samonds and Bonds, 2004; Samonds et al., 2004).
4. Does synchrony have a role in visual perception?
As mentioned earlier, in the visual system at the level of retinal ganglion cells and LGN cells action potentials are already highly synchronized. This early form of spike synchronization is thought to be mediated by the large number of gap junctions that remain even in adult animals at these levels and extensive convergence and divergence of inputs to the LGN (Meister 1995; Alonso et al., 1996). There has been great debate, however, as to the role of this synchronous firing at subsequent stages in the visual system. There are two general hypotheses regarding the generation and use of neuronal synchrony in the visual cortex. The first hypothesis, termed the “Temporal Binding Hypothesis,” suggests that spike synchrony and global synchrony in the form of oscillations bind stimulus features through an internally-generated mechanism (for review see von der Malsburg, 1982; Crick and Koch, 1990; Singer, 1993; Eckhorn, 1994, Engel and Singer, 2001). The second hypothesis suggests that spike synchrony is generated by the spatio-temporal properties of a stimulus and serves to provide perceptually relevant information about the stimulus not available from changes in firing rates (Samonds et al., 2003; Samonds and Bonds, 2004; Samonds et al., 2004; Samonds and Bonds, 2005; Biederlack et al., 2006).
4.1. Does neuronal synchrony mediate feature binding?
The Temporal Binding Hypothesis was first proposed by von der Malsburg (1982) and has since received much experimental attention (for review see Crick and Koch, 1990; Singer, 1993; Eckhorn, 1994, Engel and Singer, 2001). This hypothesis sought to explain how objects are identified and represented throughout the visual system. Components of an image are processed in parallel by distributed neurons within a visual area and across many of the more than 30 visual areas that have been proposed to exist in primates (Felleman and Van Essen, 1991). The Temporal Binding Hypothesis suggests that distributed neurons representing the same object have to be tagged and demarcated from neurons representing other objects in the visual field. These bound neurons were termed dynamic assemblies and the mechanism of tagging the neurons was suggested to be synchrony. Oscillations were proposed to be a mechanism of entraining membrane potential fluctuations and thus generating spike synchrony, especially over long distances. In addition to grouping the responses of these neurons, the correlated firing of cells within an assembly also was proposed as a mechanism to promote processing at higher levels in the visual hierarchy. The proponents of this hypothesis suggested that binding requires a coordinated interplay between bottom-up and top-down mechanisms. The hypothesized bottom-up mechanism was the generation of synchrony encoding Gestalt properties of the stimulus, such as continuity, vicinity and coherent motion (Koffka, 1935). Top-down mechanisms were proposed to synchronize the distributed neurons representing the same object via stimulus-dependent oscillatory synchronization (for review see von der Malsburg, 1982; Crick and Koch, 1990; Singer, 1993; Eckhorn, 1994, Engel and Singer, 2001).
Studies examining the ability of correlated firing to bind distributed neurons representing the same objects have primarily utilized texture segmentation and feature grouping tasks (Lamme and Spekreijse, 1998, Castelo-Branco et al., 2000; Roelfsema et al., 2004; Palanca and DeAngelis, 2005). Psychophysical studies show that in addition to spatial cues, the brain may use temporal difference cues as small as 3–5 ms to differentiate figure from ground (Ramachandran, 1991; Fahle, 1993; Leonards et al., 1996), suggesting that a neural code based on precise spike timing in the millisecond time scale binds neurons representing features of the same object. Finding evidence for such scene segmentation reflected by the synchronization of neural responses, however, has proven to be difficult and controversial.
The first physiological data supporting scene segmentation by synchrony in an awake animal were generated by Engel et al. (1991). When neurons with overlapping receptive fields were stimulated with a contiguous moving bar, their collective responses were synchronized. When two bars moving in different directions were presented, however, the neurons divided into two separate oscillatory synchronized assemblies based on the orientation preferences of the neurons within each assembly. Neurons were synchronized with other neurons within the same assembly but not with neurons belonging to the other assembly. Similar results were obtained in macaque area MT, where oscillations were demonstrated to have similar stimulus-specific features (Kreiter and Singer, 1996). Other studies, however, have failed to demonstrate a relationship between scene segmentation rules and correlated firing. The general finding from the latter is that during scene segmentation tasks the amount of correlated firing between cells is not significantly different depending on whether the neurons are encoding features of the same or different objects (Lamme and Spekreijse, 1998; Dakin and Bex, 2002; Roelfsema et al., 2004; Palanca and DeAngelis, 2005; van der Togt et al., 2006).
A good model for studying the role of synchrony in binding stimulus features utilizes plaid stimuli. These stimuli may be perceived either as two gratings sliding on top of each other (component motion) or as a single pattern that moves in a direction that is intermediate to the orientations of the two gratings that compose it (pattern motion). The type of motion perceived depends on the transparency of the component gratings (Adelson and Movshon, 1982; Stoner et al., 1990; Smith and Harris, 1991). A few studies have attempted to correlate synchrony with the type of motion perceived. A positive test for binding by synchrony would show two different coherent oscillatory assemblies for component motion and one large synchronized oscillatory assembly for pattern motion (Castelo-Branco et al., 2000; Thiele and Stoner, 2003; Palanca and DeAngelis, 2005). This was indeed observed by Castelo-Branco et al. (2000) in areas 18 and the posteromedial lateral suprasylvian area (PMLS) of the anesthetized cat. Area PMLS has neurons with similar properties to those in area MT of monkeys. These observations, however, have not been reproduced in area MT of the awake macaque monkey, where pattern motion elicits significantly less synchrony than component motion (Thiele and Stoner, 2003). Reasons for these discrepancies are not clear but most likely reflect the state of the animal. One thing that is well understood concerning spike and oscillatory synchrony is that these forms of correlated activity are very state-dependent (Fries et al., 2001; Thiele and Stoner, 2003; Palanca and DeAngelis, 2005; van der Togt et al., 2006). In the awake behaving subject it is clear that different cognitive states and even different task demands can produce differences in the degree and type of synchrony/oscillatory activity seen. State dependent effects need to be taken into account when analyzing such data.
A general limitation of many of the above-mentioned feature grouping studies is that these studies examined changes in correlation between pairs of neurons with overlapping or collinear receptive fields. Such receptive fields have been shown to share more extensive horizontal connections, increasing the chances of detecting correlated firing (Gilbert and Wiesel, 1983; Rockland and Lund, 1983; Kisvarday et al., 1997). By sampling more broadly and using stimuli with partially occluded objects, Palanca and DeAngelis (2005) evaluated the potential roles for spike synchrony and oscillations as mechanisms of feature binding in area MT of the awake macaque monkey. Interestingly it was shown that spike synchrony shows little dependence on feature grouping, but gamma band oscillations are significantly stronger when features are grouped. Nevertheless, the change in oscillatory activity was small compared to the variability of spike synchrony. In addition, the significant oscillatory effect was abolished when stimulus differences near the receptive fields were eliminated using partial occlusion. These findings led the authors to conclude that spike synchrony and oscillatory synchrony are not a general mechanism of feature binding (Palanca and DeAngelis, 2005).
Recently it has been shown that success in a figure/ground discrimination task is correlated with oscillatory synchrony dynamics in macaque V1 during stimulus onset (van der Togt et al., 2006). Before stimulus onset broad-peak correlations are observed that change to thin-peak correlations after stimulus onset. It was shown that the magnitude of this stimulus-dependent decrease in synchrony correlates with success in the task. The authors suggest this desynchronization reflects a change from loose and global neuronal interactions towards a finer temporal and spatial scale of neuronal interactions and that such changes are required for success in a figure/ground discrimination task (van der Togt et al., 2006). The finer neuronal interactions could be those produced by stimulus encoding neural assemblies whose spikes synchronize in response to the appearance of the stimulus (see later in this chapter). Nonetheless, these studies suggest that instead of being involved in binding stimulus features, oscillatory synchrony is more likely related to the cognitive state before the binding occurs, such as attention.
As noted above, data exist that both support and contradict a role for neuronal synchrony in feature binding. Future studies should utilize neurons with wholly different receptive field locations and stimulus preferences as well as stimuli that are less optimal for driving the responses of individual neurons, such as natural stimuli, which reflect more realistic viewing conditions. Given the current difficulty, however, in linking correlated firing with feature binding in simple discrimination tasks, it is difficult to imagine finding a role for correlated firing in more natural viewing conditions, where distributed responses from millions of neurons may need to be precisely bound into thousands of different object representing assemblies (Shimojo et al., 1988; Stoner and Albright, 1996).
A large weakness of the Temporal Binding Hypothesis is that it attempts to describe how neural codes representing object features can be bound together without providing a mechanism for the identification of objects or a mechanism that can coordinate synchrony and oscillation dynamics across distributed networks, producing these object-encoding assemblies (for review see von der Malsburg, 1982; Crick and Koch, 1990; Singer, 1993; Eckhorn, 1994, Engel and Singer, 2001). Oscillatory synchronization was proposed as a mechanism of synchronizing spike trains of distributed neurons (for review see von der Malsburg, 1982; Crick and Koch, 1990; Singer, 1993; Eckhorn, 1994, Engel and Singer, 2001). Although oscillations are modulated by top down influences, such as attention, their prevalence as well as ability to code perceptually relevant information is not well understood (Ghose and Freeman, 1992; Young et al., 1992; Bair et al., 1994; Gray and Viana Di Prisco, 1997, Samonds and Bonds, 2005). Proponents of the Temporal Binding Hypothesis have argued that the object recognition and synchrony generation occur through a coordination of bottom-up and top-down mechanisms, however, again these mechanisms, as well their interactions, have been poorly-defined and experimental evidence is inconsistent (for review see also von der Malsburg, 1982; Crick and Koch, 1990; Singer, 1993; Eckhorn, 1994, Engel and Singer, 2001).
4.2. Spike synchrony provides information on stimulus detail
The second hypothesis concerning the role of neuronal synchrony in visual processing suggests that spike synchrony (generated by the spatio-temporal properties of a stimulus) serves to provide perceptually relevant information about the stimulus (Samonds et al., 2003; Samonds and Bonds, 2004; Samonds et al., 2004; Kohn and Smith, 2005; Samonds and Bonds, 2005; Biederlack et al., 2006). Spike synchrony is suggested to work in conjunction with changes in firing rate, although each likely provides different types of information regarding a visual stimulus. This is not simply a corollary of the Temporal Binding Hypothesis but argues that spike synchrony between local populations can very effectively group cells in networks based on the natural temporal correlations that occur in viewed images since the parts of objects move together and have continuous contours. Data generated to support this hypothesis include studies showing that spike synchrony between different pairs of cells in V1 of the cat varies depending on whether they represent components of common contours. Thus, any individual cell can synchronize with different partners in the network depending on the stimulus components (2004; Samonds et al., 2004; Samonds and Bonds, 2005, Samonds et al., 2006).
Early studies of synchrony looked at the functional and anatomical correlates of spike and oscillatory synchrony. Both types are strongly dependent on neuronal proximity and feature preference as well as on the configuration of stimuli (Desimone et al., 1985; Gray et al., 1989; Engel et al., 1990; Kreiter and Singer, 1992). It was also shown that spike synchrony and oscillatory synchrony reflect Gestalt criteria (continuity, vicinity and common motion) (Koffka, 1935). Changes in these criteria are not necessarily reflected by changes in firing rate (Gray et al., 1989; Engel et al., 1991).
Many studies of neuronal synchrony also defined a correlation between the degree of synchrony and the similarity of orientation preference between two cells in V1 (Gray et al., 1989; Engel et al., 1990; Engel et al., 1991; Kohn and Smith, 2005). Recent studies, however, have provided a better understanding of the potential role of spike synchrony in orientation discrimination. By using information-theoretic analyses, it was shown that changes in firing rate provide the information necessary for discrimination of coarse orientation differences, with little information gained from the cooperative activity of neurons. In contrast, cooperative activity encodes most of the information required for fine angle discrimination, with no significant information gained from changes in firing rates (Samonds et al., 2003; Samonds et al., 2004). It was also demonstrated that increasing the number of cells in an assembly increases the amount of information coded (Samonds and Bonds, 2005). These studies provide a biologically plausible mechanism for hyperacuities (Westheimer, 1981).
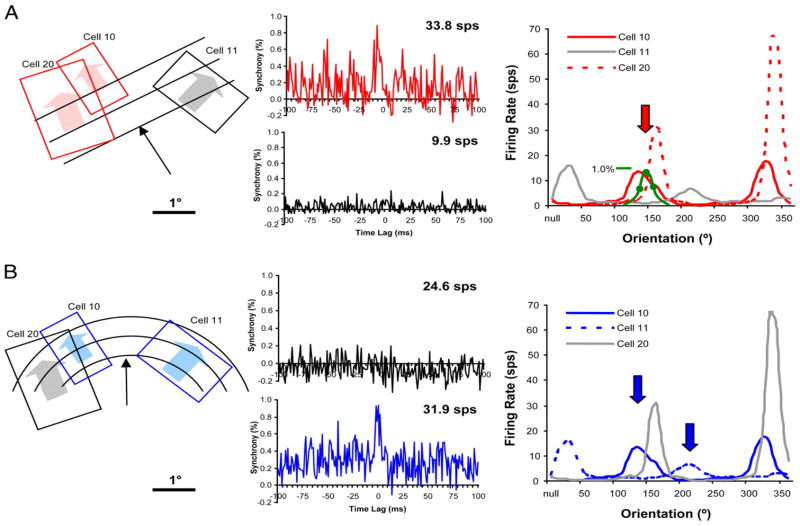
Recently, encoding of contours by spike synchrony has been demonstrated for cocircular contours (Samonds et al., 2006). Spike synchrony was found between cells with largely different orientation preferences when their receptive fields were stimulated simultaneously by the edge of a circle. The degree of synchronization was found to be orientation and curvature-dependent and demonstrated greater selectivity for cocircular contours than changes in firing rate (Fig. 2). The authors suggested that this mechanism serves to shape extrastriate contour response selectivity (Samonds et al., 2006).
Figure 2.
Examples of synchrony dynamics for rings and gratings. (A) When two cells with collinearly-aligned receptive fields (cells 10 and 20) were stimulated with a grating, the spikes of these two neurons were synchronized. These neurons were not, however, synchronized with cell 11, whose receptive field is not collinearly aligned with the other two. (B) When cells with cocircularly-aligned receptive fields (cells 10 and 11) were simultaneously stimulated with a concentric ring, spikes of the two cells were synchronized, but are not synchronized with cell 20, whose receptive field was not cocircularly aligned with cells 10 and 11. Significant stimulus-dependent differences were not observed in the firing rates. These results indicate that spike synchrony may be sensitive to cocircular contours in a manner that firing rate is not. (Adapted from J. Neurophysiol., 95 (2006) 2602–2616 Fig. 11 with permission from the publisher)
In addition, recent evidence from our lab indicates that spike synchrony is dependent on spatial frequency in a manner that firing rate is not. The amount of spike synchrony present in bush baby V1 is greater at higher spatial frequencies. The spike synchrony at these higher spatial frequencies is better at discriminating fine differences in spatial frequency than is firing rate. An attractive hypothesis is that spike synchrony has evolved as an important strategy for coding for stimulus structural detail (Jermakowicz et al., 2006).
Biederlack et al. (2006) argue that spike synchrony and changes in firing rate have different roles in coding for perception of stimulus brightness in the cat. The perceived brightness of a center grating was modulated by either changing the orientation of a surround grating relative to the center or by changing the phase of the surround grating relative to the center grating. Parallel multiunit responses were collected from V1 cells whose receptive fields focused on the center grating. Changing the surround orientation produced significant increases in firing rate without affecting spike synchrony between the units. On the other hand, Altering the surround grating’s phase relative to the center grating increased spike synchrony between the units without affecting firing rates. These results suggest that separate codes, one based on changes in firing rate and the other based on spike synchrony, may code perceived brightness depending on how the illusion is induced. It is not known, however, how the different stimulus manipulations lead to the separate changes in neuronal response properties.
A limitation of the spike synchrony hypothesis is that it has not yet been demonstrated that extrastriate areas are sensitive to the information encoded by correlated activity in lower visual areas. Thus, although we have demonstrated that stimulus-dependent spike synchrony in lower areas correlates with driving efficacy of spikes between areas, the perceptual relevance of this relationship is unclear. Also, the stimulus-dependent network properties that generate the perceptually relevant synchrony need to be elucidated. These properties have been hypothesized to include bursting, convergence and divergence, oscillations and chaos (Samonds et al., 2005), however, likely involve many other features. A role for these and other mechanisms in generating the relevant correlated activity needs to be determined.
5. What is the role of synchrony in other brain systems?
5.1. The auditory system
Auditory neurons have long been known to be able to produce precise stimulus-dependent responses (Moiseff and Konishi, 1981; Hill et al., 1989; Oertel, 1999). In addition, the ability of auditory brainstem neurons to act as coincidence detectors that can detect the interaural time differences (ITDs) between spikes from the two ears and deduce sound source location was one of the first examples demonstrating that spike synchrony may code useful information (Moiseff and Konishi, 1981; Yin and Kuwada, 1983). These neurons, which are in the medial superor olive of the brain stem, are morphologically unique and highly sensitive to ITDs. They have been exhaustively studied as models of coincidence detectors. Investigations of these neurons suggest that several factors including their bipolar shape, the site of spike generation, receptor expression and electrical properties of soma and axon have been optimized for the precise detection of coincident spikes (Kuba et al., 2005; Ashida et al., 2006; Kuba et al., 2006).
The role of neuronal synchrony in auditory perception has been studied at several different levels of the auditory system. Peripheral auditory receptors respond very precisely to auditory stimuli. The responses of these receptors are faithfully synchronized to the onset of sounds and the neuronal population responses to different sounds closely mimic the temporal patterns of the stimuli (reviewed by Mason and Faure, 2004). Using information-theoretic analyses, Machens et al. (2001) studied the ability of single insect auditory receptors and of populations of these receptors to encode sounds. They found that although responses of single receptors are sensitive to temporal properties of the stimulus, using population responses greatly increased the sensitivity of the response to near behavioral levels.
Woolley et al. (2006) elegantly examined synchrony among neurons in the songbird midbrain in response to natural (songs) vs. artificial (noise) sounds. In response to the noise stimuli, midbrain neurons’ spikes are not temporally precise and not significantly synchronized. Natural songs, however, elicited very temporally precise spikes synchronized to the stimulus. This produced powerful and temporally precise synchronized population responses to the songs. No significant differences were found among the firing rates of the neurons in response to the different stimuli, including songs. The authors suggest that the increase in temporal precision and synchrony of spikes not only serves to increase the precision at which sounds can be encoded, but also serves to increase the likelihood of these responses being processed at later stages in the auditory system. What remains to be investigated and is not known is why the different patterns of sounds produce such different neuronal responses.
As is the case with visual cortex neurons, synchrony among neurons in the auditory cortex of mammals is strongly dependent on the similarity of receptive field properties, specifically their characteristic frequencies, intensity thresholds and the temporal evolution of their responses (Brosch and Schreiner, 1999). Tomita and Eggermont (2005) recently demonstrated that synchronous activity among neuron pairs in the auditory cortex of the cat may contain information not available from firing rates. Using multiple single unit recordings, they determined the spectro-temporal receptive fields (STRFs) of groups of primary auditory cortex neurons. The STRFs of coincident spikes were smaller than their single-unit counterparts, suggesting that synchronous spikes could serve to sharpen resolution in the spectro-temporal domain. These same authors also distinguished between spikes that contributed to the STRF (IN-STRF) and spikes that did not contribute (OUT-STRF; proposed to be background noise) and looked at correlations between these groups among different neurons under different stimulus conditions. During stimulation, correlations among IN-STRF spikes where increased, while correlations among OUT-STRF spikes were decreased. The authors suggest that this decrease in correlation between background spikes occurs because during silence auditory neurons are organized into a large synchronous assembly, but with stimulus onset this large assembly is broken-down into many smaller stimulus-specific synchronous assemblies or networks.
5.2. The Olfactory System
Some of the most direct evidence for a functional role of synchrony in perception comes from studies of the olfactory system. It is known that the olfactory bulb expresses prominent oscillatory synchrony during odorant stimulation (Adrian, 1950; Rall and Shepherd, 1968; Freeman; 1972). Neurons in the insect olfactory bulb engage in synchronous oscillatory activity around 20–35 Hz (Laurent and Naraghi, 1994). Information-theoretic studies have demonstrated that changes in oscillatory activity in the insect olfactory bulb are a reliable mechanism for signaling changes in odor content (Laurent, 1996; Laurent et al., 1996; MacLeod and Laurent, 1996; Wehr and Laurent, 1996). Changes in correlated firing are also associated with sensory learning. Repeated exposures to an odor are associated with decreased firing rate modulation, but increased synchrony and oscillatory amplitude in the insect olfactory bulb (Stopfer and Laurent, 1999). Upon pharmacological desynchronization (without associated changes in firing rate) of olfactory bulb responses, odor discrimination deteriorates (Stopfer et al., 1997) and cells at higher processing stages lose some of their odor specificity (MacLeod et al., 1998). These studies provide a very strong link between synchrony and perception of sensory stimuli.
Concepts derived from the insect olfactory system have been extended to vertebrate species, where it has also been shown that correlated firing also can provide additional odorant information beyond that obtained strictly from firing rates and may be involved in olfactory learning (Friedrich et al., 2004; Zochowski et al., 2005). In addition, Nusser et al. (2001) demonstrated that alteration of GABAergic inhibition in the mouse olfactory bulb alters the spatiotemporal odorant code and correlates with impaired odor discrimination. Beyond their physiological and behavioral contributions these studies have also been instrumental in determining the anatomical substrates that support synchrony and coincidence detection (see section on anatomy).
5.3. The Somatosensory and Motor systems
Several studies have looked for a potential role for neuronal synchrony in information coding in the somatosensory and motor systems. Stimulus-dependent oscillatory and non-oscillatory synchrony is prominent in the primary and secondary somatosensory cortices (Murthy and Fetz, 1992). Although both forms of synchrony are common among these neurons, they do not appear to be related to each other. Most synchronized neurons do not display oscillations and the pairs that do typically do not oscillate at the same frequency (Roy et al., 2001). Bruno and Sakmann (2006) suggest that the responses in somatosensory cortex (SI) are driven by synchronous thalamocortical inputs. These inputs are a minority of the inputs onto SI neurons and they are weak at driving the SI neurons individually, but because these inputs are highly convergent and synchronous, they are efficiently able to generate SI responses. It is noteworthy that similar proposals have been made about thalamocortical inputs in the visual system (Alonso et al., 1996). As in the somatosensory system the thalamus provides less than 10% of the synapses on recipient neurons in the visual cortex so mechanisms that enhance this weak input must exist.
Synchrony, however, does not always seem to provide a better code than firing rate in the somatosensory system. For example, Alloway et al. (2002) examined the role of synchrony in coding for stimulus direction in cat secondary somatosensory cortex (SII). They ran a focal air jet along the distal forelimb of the cat and looked at coding of direction by the firing rates of individual cells and also by the synchrony among cell pairs. Whereas 63.4% of cells displayed a direction preference in their firing rates, only 14.2% of synchronized cells displayed a direction preference in their correlated activity, suggesting that synchronous activity does not efficiently code for stimulus direction, at least not in the cat somatosensory cortex.
In some cases, however, synchrony does appear to provide information in cat SII. Steinmetz et al. (2000) demonstrated that the amount of synchrony between SII area neurons is strongly dependent on the difficulty of a visual-tactile discrimination task. They suggest that the synchrony may be a method of regulating attentional selection (see also below). Clearly, at this stage too little is known about the role of synchrony in the somatosensory system to come to any general conclusions as to its role.
Synchrony and oscillations are also prominent in the motor cortex, where they are modulated at different phases of motor tasks (Murthy and Fetz, 1996a,b; Baker et al. 2003) and have been implicated in motor learning (Cohen and Nicolelis, 2004; Kargo and Nitz, 2004; Paz and Vaadia, 2004). Highly-regulated correlations are considered to be important in the proper coordination of distal muscles, whose inputs are synchronized with pyramidal neurons in the primary motor cortex (MI) (Baker et al., 1999, 2003).
Early studies of spike synchrony and oscillations in MI addressed the “association hypothesis”, the equivalent of the “binding hypothesis” in the visual system (Murthy and Fetz, 2002). This hypothesis suggested that sensorimotor oscillations could facilitate associations between cells involved in the same task. Although it had been demonstrated that motor neurons involved in the same task had a slightly higher probability of synchronizing with each other compared to neurons not involved in the same task, this difference was found not to be statistically significant and this hypothesis was soon abandoned (Murthy and Fetz, 1996 a,b).
Recently, however, Brecht et al. (2004) showed that the amplitude and direction of saccadic eye movements depend not only on the site and amplitude of activation of the superior colliculus, but also on the degree of spike synchrony between driving neurons. The latter study was performed by inserting microelectrodes and simultaneously stimulating different collicular neurons with varying degrees of synchronicity. With synchronous inputs the resulting saccade vector was the average of the vectors resulting from stimulating each site alone, just as in normal behavior. In contrast, when different neurons were stimulated asynchronously, the resulting saccade was the sum of the saccades obtained from stimulating each site alone (Brecht et al., 2004). Thus, the authors showed a behavioral correlate of increased synchrony in the superior colliculus, demonstrating that some types of movements can be sensitive to synchronous vs asynchronous inputs.
Narayanan et al. (2005) used an information-theoretic approach to quantifying synergy and redundancy among neurons within neuronal ensembles in rat MI during simple motor tasks. If neurons cooperate synergistically, then the information gained from their population activity is more than the sum of the information gained from the activity of the individual neurons. The opposite is true for redundancy. Although synergy and redundancy do not specifically refer to synchrony, they refer to the information that can be gained from cooperative firing, much of which can be due to synchronous firing. In smaller ensembles (two neurons) in rat M1 the cells often displayed synergistic interactions, but when the ensembles were large (eight neurons) mostly redundant interactions were observed, indicating that neurons could be removed from the ensemble without necessarily compromising information. These results suggest that MI neurons do not function as independent encoders of behaviorally relevant information, but work together as a coordinated assembly. It is not clear why the neuronal interactions in this study were observed to be mostly redundant, whereas other studies of neuronal cooperation found mainly synergistic interactions (Maynard et al. 1999; Samonds et al., 2004). The redundancy among MI neurons has been suggested to exist in order to overcome the highly diverse corticospinal neuron onset latencies (Meijers and Eijkman, 1974) or to allow for increased efficiency in controlling functionally-related sets of muscles (Narayanan et al., 2005), however, further studies need to be performed to determine the functional relevance of this redundant activity and what the specific role of synchrony is in these interactions.
As mentioned in the Introduction, the first physiological data suggesting a role for precisely timing in neural coding were published by Sir Charles Sherrington (1906, 1941). He sectioned cat spinal cords and examined population activity during coordinated locomotion. His data suggested that locomotion may involve the interaction of precisely correlated cell assemblies. Since these pioneering studies, our understanding of these networks has greatly increased. At the spinal cord these models are especially attractive because spike timing has been analyzed at many levels, from genetic to behavioral. It is known that local neuron assemblies, called central pattern generators (CPGs), control the precise, rhythmic timing of cells (motor neurons, muscle fibers, interneurons, etc.) involved in generating motor patterns (For review see Schomburg et al., 1998; Kiehn, 2006). When locomotion is initiated the many components of the CPGs are recruited into a functional unit forming one rhythmic network (Lafreniere-Roula & McCrea, 2005). Many players are involved. For example, there are excitatory glutaminergic interneurons projecting to local and distal motor neurons that generate rhythmic and precise bursting activity (Grillner, 2003). These interneurons as well as the other elements in these CPGs have been well characterized structurally and functionally. Transient and sustained phases of synchronous and asynchronous responses are common features of these networks. Disruptions of the temporal patterning of this activity lead to severe motor deficits (For review see Hultborn et al., 1998; Matsuyama et al., 2004; Cangiano and Grillner, 2005; Kiehn, 2006). These studies collectively suggest that the spinal cord architecture is designed to maintain the precise spike timing of population responses and that spike train correlations are a fundamental mechanism of neural coding in the spinal cord.
6. Synchrony and higher cognitive processes
6.1. Attention
Several ideas regarding the relevance of spike synchrony and oscillations in the brain require that in addition to being sensitive to sensory stimulation, that such correlated firing be modifiable by cognitive processes that bias the system, such as attention (for review see Singer, 1993; Eckhorn, 1994; Singer and Gray, 1995; Engel et al., 2001). Stimulus dependent gamma oscillations are induced by stimulation of the mesencephalic reticular formation, a region heavily implicated in attention (Munk, 1996). This effect was shown to be due to the activation of the cholinergic system (Rodriguez et al., 2004), suggesting that that stimulation at the level of the brainstem may drive the interconnected groups of cholinergic neurons that have axons distributed throughout the brain. The correlation of these processes potentially linked to attention with gamma oscillations suggests that attention may be capable of regulating oscillatory synchrony, although the link is admittedly indirect. Additionally, many recent studies have described the attentional dependency of neuronal synchrony (for review see Engel et al., 2001; Fries et al., 2001; Niebur et al., 2002; Ward, 2003; Tallon-Baudry, 2004). For example, attention has been shown to modulate oscillatory synchronization in the fourth visual area (V4) of macaque monkeys, an area important in the hierarchy of visual areas concerned with object recognition (Fries et al., 2001; Tiesinga et al., 2004; Taylor et al., 2005; Womelsdorf et al., 2006). The latter studies showed that there is an increase in gamma frequency oscillations and decrease in lower frequency (<17 Hz) oscillations between two neurons representing an attended object compared to neurons representing a distracter. Errors, indicated by inappropriate selection of the distracter, were preceded by an oscillatory shift from neurons representing the target to neurons representing the distracter (Fries et al., 2001; Taylor et al., 2005; Tiesinga et al., 2004; Womelsdorf et al., 2006). It has been suggested that this increase in coherence is a reflection of an increase in synchrony between local inhibitory interneurons (Tiesinga et al., 2004).
Several studies using magnetoencephalography (MEG) and electroencephalography (EEG) have recently added to evidence of a link between oscillatory synchrony and attention. Bauer et al. (2006) described conditions where subjects receive tactile stimulation of both index fingers. Cueing attention to one finger caused an increase in gamma band activity. Analogous to the above-mentioned experiments concerning monkey V4, lower frequency oscillations were reduced under conditions of increased attentional load.
Recently, Gross and colleagues (2004) elegantly demonstrated a link between oscillatory synchronization and success in the attentional blink paradigm, which tests subjects’ abilities to perceive two objects presented a short interval (about 100 ms) apart. Using MEG they studied transient long-range inter-areal oscillatory synchronization between frontal, temporal and parietal lobes, regions identified to be key players in the attentional modulation of visual processing (Desimone and Duncan, 1995; Marois et al., 2000; Corbetta and Shulman, 2002). Their results show that oscillatory synchronization between these areas in the beta range (around 15 Hz) was greater during the presentation of the stimuli when the second object of the attentional blink task was consciously detected. They hypothesized that the higher degree of oscillatory synchrony was associated with better use of attentional resources (Gross et al., 2004).
Vidal et al. (2006) hypothesized that if oscillatory synchrony is useful in visual attention, then different attentional tasks should produce different oscillatory effects. To test this idea they presented subjects with visual tasks that involved two different components: 1) attention to the manipulation of grouping properties, and 2) focusing of attention to memorize part of the display. In the task, eight differently-oriented bars were presented and the subjects had to focus on either four or eight bars (grouping component). The bars then briefly disappeared and reappeared, after which the subjects had to decide whether the orientation of any of the bars had changed (memory component). Responses were recorded by MEG. They found that altering the difficulty of the grouping component of the task increased the power of the high (70–120 Hz) gamma-band, while changing the difficulty of the memorization component increased the power at the lower gamma range (44–66 Hz). These data suggest that oscillatory synchrony may play a role in coordinating activity over long distances and that different types of complex tasks could utilize oscillatory synchronization at different frequencies.
Oscillatory synchrony has also been strongly implicated in access to consciousness (Srinivasan et al., 1999; Palva et al., 2005; Melloni et al., 2007). Melloni et al. (2007) recently examined the role of oscillations in conscious perception by examining responses to visible and invisible words in a delayed matching to sample task. Both visible and invisible words generated local gamma oscillations, however, only the visible words yielded transient gamma-band synchrony between distant areas in the brain. The global theta-band signature was also different for the two different responses. These results suggest that transient long-rage oscillatory synchrony is required for access to consciousness.
6.2. Memory
Different types of memory require careful coordination of cell responses over distributed networks. Thus, if coordinated firing mediates long-distance neuronal communication, it is likely to be involved in both memory formation and recall. It is already known that spike timing is critical to the creation of synaptic models of memory since it is essential to the production of either long term potentiation (LTP) or long term depression (LTD) (see above). Additionally, several studies have directly linked oscillatory synchrony and memory. Fell et al. (2001, 2003) studied declarative memory formation (memory for facts and events) in humans while recording depth-EEG from within the medial temporal lobe. Successful memory formation was accompanied by transient increases in gamma and theta oscillatory synchrony between the hippocampus and neighboring rhinal cortex, structures known to be involved in memory formation. These changes in oscillatory frequency were not observed in cases where the memory formation was not successful. These studies suggest that declarative memory formation in the medial temporal lobe is mediated by transient oscillatory synchrony, linking the hippocampus with other areas presumably involved in laying down these memories.
Oscillatory synchrony also has been implicated in memory recall. When searching for a target, an attended stimulus typically has to be matched with a template in working memory. If oscillatory synchrony is a long-range communication mechanism, stimuli that match a memory template during a search task should evoke stronger gamma oscillations than those that don’t. This relationship was observed by Debener et al. (2003) using EEG measures in human subjects when they performed an auditory search task. Subjects were presented auditory stimuli (template) and these had to be remembered to be compared to subsequently-presented sounds. The auditory stimuli that matched the template led to significantly more gamma-band synchrony than those sounds that didn’t match the template.
Oscillatory synchrony also has been implicated in long-term memory of visual stimuli. Stimuli for which subjects have a long-term memory representation lead to significantly greater gamma oscillations in the occipital cortex than similar stimuli that are not in long-term memory (Herrmann et al., 2004). Some studies also have reported an increase in gamma oscillations in the frontal lobe during long-term memory retrieval. It has been suggested that these frontal cortex oscillations mediate the gamma-band oscillatory synchrony in the occipital cortex (Tallon-Baudry et al., 1997; Struber et al., 2000), however more detailed analyses are needed to confirm this finding.
Recently, several findings have strongly implicated theta rhythms in memory processes. Invasive recordings in patients suggest that theta-band oscillations are implicated in spatial navigation (Caplan et al., 2001, 2003), working memory (Raghavachari et al., 2001; Rizzuto et al., 2003) and episodic memory (Sederberg et al., 2003). Work by Guderian and Duzel (2005) indicate that large-scale synchrony mediated by theta oscillations are involved in recollection in humans. Using MEG recordings, theta oscillations were seen in human subjects who were required to perform a face recognition task with pictures of faces they had seen and new faces. Cases where the subjects successfully recognized faces were associated with increased theta activity in a distributed network that included prefrontal, mediotemporal and visual areas in occipital cortex. The authors of this study suggest that the theta activity mediates a dynamic link between the hippocampus and distributed neocortical assemblies which have been implicated in memory (Treves and Rolls, 1994; McClelland et al., 1995; Rolls, 2000).
Several additional studies have linked memory and other frequencies of oscillatory synchrony, including alpha and beta oscillations (Tallon-Baudry et al., 2001, Tallon-Baudry et al., 2004; Palva et al., 2005; for review see Hermann et al., 2004). A general finding of these studies regarding attention and memory is that different cognitive states have different spatiotemporal signatures of oscillatory activity. In addition to showing that synchrony is involved with these different cognitive processes, the data suggest that oscillatory synchrony may be the mechanism by which the coordination of distributed assemblies of neurons occurs during these processes. Given that much of the data on oscillations has been collected in non-invasive studies, however, it is still unclear how oscillations relate to spike synchrony under conditions where there are major demands on attention or memory. What evidence we do have, however, argues strongly that both spike synchrony at the local level and oscillatory activity at the global level are necessary for neurons to “work together” in coding relevant information at several different levels.
7. Conclusions and Remaining Questions
Our assignment in this essay was to explore how the concept of the neuron has changed in the century since Golgi and Cajal shared the Nobel Prize. We have argued throughout this essay that one area that has undergone a conceptual change concerns the function of neurons. Although neurons are clearly still seen as individual units, functionally their group behavior represents more than the sum of each neuron’s individual parts, and more than would be predicted based on each neuron’s ability to produce trains of action potentials. The important point is that, although one can identify the anatomical boundaries of neurons in space as Cajal and Golgi both beautifully demonstrated, neuronal function is a group effort. New properties emerge from neurons “working together,” in Golgi’s words, in specific networks. One could argue that this idea is not a new perspective and that the philosophers and early psychologists made this point years before Cajal or Golgi ever described a neuron anatomically. The difference is that today we have the technological means to actually test this idea by eavesdropping on the behavior of individual neurons and asking about how they behave dynamically in small or large groups. What such eavesdropping shows is that a neuron’s function must be defined relative to the group to which it is dynamically connected.
In this brief overview we defended the position that emergent properties of neuronal networks are defined by synchrony. This synchrony can involve small local networks engaged in spike synchrony or large global networks that appear to communicate via different oscillation frequencies. Evidence to support this showed that spike synchrony can code information that is independent of firing rate and that, especially in the visual system, such coding appears to add information about spatial detail. Evidence from other sensory systems like the insect olfactory system, also suggests that spike synchrony between neurons can code additional information about stimulus quality, information not available in the firing rate of individual neurons.
Although the exact relationship between spike synchrony and oscillatory synchrony still remains to be defined, oscillatory synchrony appears to be involved in coordinating long-distance neuronal communication. Processes such as attention, memory storage and memory retrieval may utilize different frequencies of oscillatory synchrony to coordinate communication among distributed networks of neurons across large brain regions such as between the cortex of the temporal and frontal lobes and the hippocampus.
At this juncture, although there is general agreement that information coding by neurons involves some sort of population code, no general consensus exists as to exactly how this works. Synchrony among local and global populations of neurons has many attractive features some of which we have reviewed here. Many questions remain to be answered before it is clear exactly how information is coded by neurons. We have listed some key remaining questions below.
What are the relative roles of direct stimulus properties and internal neural mechanisms in generating spike synchrony?
What cellular and network mechanisms generate and propagate a stimulus-dependent code based on spike synchrony?
How do firing rate and synchrony work together to code information
Do membrane integration constants vary dynamically in a manner that can simultaneously allow codes based on synchrony and firing rate?
How dependent are spike synchrony and oscillatory synchrony on each other?
What is the origin of different oscillation frequencies? Are they functionally relevant or are they an epiphenomenon of neuronal communication that can eventually be accounted for using electrophysiological and simulation studies?
How are spike synchrony or oscillations affected by anesthesia and the arousal state of the system?
Are the time scales of spike synchrony the same in local networks at different levels of the nervous system?
Are there general rules that govern the type of information that is provided by different types of temporal codes such as firing rate, spike and oscillatory synchrony?
How does anatomy constrain temporal coding at different levels of the nervous system?
Acknowledgments
We are grateful to Dr. Gopathy Purushothaman, Dr. Xin Chen, Dr. David Royal, Ilya Khaytin and Julia Mavity-Hudson for helpful comments on earlier versions of this chapter. In addition, we extend our gratitude to two anonymous reviews for their helpful comments. Supported by National Institutes of Health grants EY01778 (VAC) and Core Grants EY08126 and HD15052.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Abeles M. Role of the cortical neuron: integrator or coincidence detector? Isr J Med Sci. 1982;18:83–92. [PubMed] [Google Scholar]
- 2.Adelson EH, Movshon JA. Phenomenal coherence of moving visual patterns. Nature. 1982;300:523–525. doi: 10.1038/300523a0. [DOI] [PubMed] [Google Scholar]
- 3.Adrian ED. Olfactory reactions in the brain of the hedgehog. J Physiol. 1942;100:459–473. doi: 10.1113/jphysiol.1942.sp003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adrian ED. The electrical activity of the mammalian olfactory bulb. Electroencephalogr Clin Neurophysiol. 1950;2:377–388. doi: 10.1016/0013-4694(50)90075-7. [DOI] [PubMed] [Google Scholar]
- 5.Aertsen AM, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of “effective connectivity”. J Neurophysiol. 1989;61:900–917. doi: 10.1152/jn.1989.61.5.900. [DOI] [PubMed] [Google Scholar]
- 6.Agmon-Snir H, Segev I. Signal delay and input synchronization in passive dendritic structures. J Neurophysiol. 1993;70:2066–2085. doi: 10.1152/jn.1993.70.5.2066. [DOI] [PubMed] [Google Scholar]
- 7.Alais D, Blake R, Lee SH. Visual features that vary together over time group together over space. Nat Neurosci. 1998;1:160–164. doi: 10.1038/414. [DOI] [PubMed] [Google Scholar]
- 8.Alloway KD, Zhang M, Dick SH, Roy SA. Pervasive synchronization of local neural networks in the secondary somatosensory cortex of cats during focal cutaneous stimulation. Exp Brain Res. 2002;147:227–242. doi: 10.1007/s00221-002-1233-3. [DOI] [PubMed] [Google Scholar]
- 9.Alonso JM, Usrey WM, Reid RC. Precisely correlated firing in cells of the lateral geniculate nucleus. Nature. 1996;383:815–819. doi: 10.1038/383815a0. [DOI] [PubMed] [Google Scholar]
- 10.Angelucci A, Levitt JB, Walton EJ, Hupe JM, Bullier J, Lund JS. Circuits for local and global signal integration in primary visual cortex. J Neurosci. 2002;22:8633–8646. doi: 10.1523/JNEUROSCI.22-19-08633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashida G, Abe K, Funabiki K, Konishi M. Passive soma facilitates submillisecond coincidence detection in the owl’s auditory system. J Neurophysiol. 2006 doi: 10.1152/jn.00399.2006. [DOI] [PubMed] [Google Scholar]
- 12.Azouz R, Gray CM. Adaptive coincidence detection and dynamic gain control in visual cortical neurons in vivo. Neuron. 2003;37:513–523. doi: 10.1016/s0896-6273(02)01186-8. [DOI] [PubMed] [Google Scholar]
- 13.Bair W, Koch C, Newsome W, Britten K. Power spectrum analysis of bursting cells in area MT in the behaving monkey. J Neurosci. 1994;14:2870–2892. doi: 10.1523/JNEUROSCI.14-05-02870.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bair W, Koch C. Temporal precision of spike trains in extrastriate cortex of the behaving macaque monkey. Neural Comput. 1996;8:1185–1202. doi: 10.1162/neco.1996.8.6.1185. [DOI] [PubMed] [Google Scholar]
- 15.Baker SN, Kilner JM, Pinches EM, Lemon RN. The role of synchrony and oscillations in the motor output. Exp Brain Res. 1999;128:109–117. doi: 10.1007/s002210050825. [DOI] [PubMed] [Google Scholar]
- 16.Baker SN, Pinches EM, Lemon RN. Synchronization in monkey motor cortex during a precision grip task. II. effect of oscillatory activity on corticospinal output. J Neurophysiol. 2003;89:1941–1953. doi: 10.1152/jn.00832.2002. [DOI] [PubMed] [Google Scholar]
- 17.Bauer M, Oostenveld R, Peeters M, Fries P. Tactile spatial attention enhances gamma-band activity in somatosensory cortex and reduces low-frequency activity in parieto-occipital areas. J Neurosci. 2006;26:490–501. doi: 10.1523/JNEUROSCI.5228-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer R, Eckhorn R, Jordan W. Iso- and cross-oriented columns in cat striate cortex: a study with simultaneous single- and multi-unit recordings. Neuroscience. 1989;30:733–740. doi: 10.1016/0306-4522(89)90165-6. [DOI] [PubMed] [Google Scholar]
- 19.Bernander O, Douglas RJ, Martin KA, Koch C. Synaptic background activity influences spatiotemporal integration in single pyramidal cells. Proc Natl Acad Sci U S A. 1991;88:11569–11573. doi: 10.1073/pnas.88.24.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry MJ, Warland DK, Meister M. The structure and precision of retinal spike trains. Proc Natl Acad Sci U S A. 1997;94:5411–5416. doi: 10.1073/pnas.94.10.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry MJ, Meister M. Refractoriness and neural precision. J Neurosci. 1998;18:2200–2211. doi: 10.1523/JNEUROSCI.18-06-02200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biederlack J, Castelo-Branco M, Neuenschwander S, Wheeler DW, Singer W, Nikolic D. Brightness induction: rate enhancement and neuronal synchronization as complementary codes. Neuron. 2006;52:1073–1083. doi: 10.1016/j.neuron.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Brecht M, Singer W, Engel AK. Amplitude and direction of saccadic eye movements depend on the synchronicity of collicular population activity. J Neurophysiol. 2004;92:424–432. doi: 10.1152/jn.00639.2003. [DOI] [PubMed] [Google Scholar]
- 24.Brosch M, Schreiner CE. Correlations between neural discharges are related to receptive field properties in cat primary auditory cortex. Eur J Neurosci. 1999;11:3517–3530. doi: 10.1046/j.1460-9568.1999.00770.x. [DOI] [PubMed] [Google Scholar]
- 25.Bruno RM, Sakmann B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science. 2006;312:1622–1627. doi: 10.1126/science.1124593. [DOI] [PubMed] [Google Scholar]
- 26.Buracas GT, Zador AM, DeWeese MR, Albright TD. Efficient discrimination of temporal patterns by motion-sensitive neurons in primate visual cortex. Neuron. 1998;20:959–969. doi: 10.1016/s0896-6273(00)80477-8. [DOI] [PubMed] [Google Scholar]
- 27.Cangiano L, Grillner S. Mechanisms of rhythm generation in a spinal locomotor network deprived of crossed connections: the lamprey hemicord. J Neurosci. 2005;25:923–935. doi: 10.1523/JNEUROSCI.2301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caplan JB, Madsen JR, Raghavachari S, Kahana MJ. Distinct patterns of brain oscillations underlie two basic parameters of human maze learning. J Neurophysiol. 2001;86:368–380. doi: 10.1152/jn.2001.86.1.368. [DOI] [PubMed] [Google Scholar]
- 29.Caplan JB, Madsen JR, Schulze-Bonhage A, Aschenbrenner-Scheibe R, Newman EL, Kahana MJ. Human theta oscillations related to sensorimotor integration and spatial learning. J Neurosci. 2003;23:4726–4736. doi: 10.1523/JNEUROSCI.23-11-04726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casagrande VA, Xu X, Sary G. Static and dynamic views of visual cortical organization. Prog Brain Res. 2002;136:389–408. doi: 10.1016/s0079-6123(02)36032-1. [DOI] [PubMed] [Google Scholar]
- 31.Castelo-Branco M, Neuenschwander S, Singer W. Synchronization of visual responses between the cortex, lateral geniculate nucleus, and retina in the anesthetized cat. J Neurosci. 1998;18:6395–6410. doi: 10.1523/JNEUROSCI.18-16-06395.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castelo-Branco M, Goebel R, Neuenschwander S, Singer W. Neural synchrony correlates with surface segregation rules. Nature. 2000;405:685–689. doi: 10.1038/35015079. [DOI] [PubMed] [Google Scholar]
- 33.Christie JM, Bark C, Hormuzdi SG, Helbig I, Monyer H, Westbrook GL. Connexin36 mediates spike synchrony in olfactory bulb glomeruli. Neuron. 2005;46:761–772. doi: 10.1016/j.neuron.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 34.Cohen D, Nicolelis MA. Reduction of single-neuron firing uncertainty by cortical ensembles during motor skill learning. J Neurosci. 2004;24:3574–3582. doi: 10.1523/JNEUROSCI.5361-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Constantine-Paton M, Cline HT. LTP and activity-dependent synaptogenesis: the more alike they are, the more different they become. Curr Opin Neurobiol. 1998;8:139–148. doi: 10.1016/s0959-4388(98)80017-2. [DOI] [PubMed] [Google Scholar]
- 36.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 37.Crick F, Koch C. Some reflections on visual awareness. Cold Spring Harb Symp Quant Biol. 1990;55:953–962. doi: 10.1101/sqb.1990.055.01.089. [DOI] [PubMed] [Google Scholar]
- 38.Crick F, Koch C. A framework for consciousness. Nat Neurosci. 2003;6:119–126. doi: 10.1038/nn0203-119. [DOI] [PubMed] [Google Scholar]
- 39.Dakin SC, Bex PJ. Role of synchrony in contour binding: some transient doubts sustained. J Opt Soc Am A Opt Image Sci Vis. 2002;19:678–686. doi: 10.1364/josaa.19.000678. [DOI] [PubMed] [Google Scholar]
- 40.Dan Y, Alonso JM, Usrey WM, Reid RC. Coding of visual information by precisely correlated spikes in the lateral geniculate nucleus. Nat Neurosci. 1998;1:501–507. doi: 10.1038/2217. [DOI] [PubMed] [Google Scholar]
- 41.Debener S, Herrmann CS, Kranczioch C, Gembris D, Engel AK. Top-down attentional processing enhances auditory evoked gamma band activity. Neuroreport. 2003;14:683–686. doi: 10.1097/00001756-200304150-00005. [DOI] [PubMed] [Google Scholar]
- 42.Desimone R, Schein SJ, Moran J, Ungerleider LG. Contour, color and shape analysis beyond the striate cortex. Vision Res. 1985;25:441–452. doi: 10.1016/0042-6989(85)90069-0. [DOI] [PubMed] [Google Scholar]
- 43.Desimone R, Schein SJ. Visual properties of neurons in area V4 of the macaque: sensitivity to stimulus form. J Neurophysiol. 1987;57:835–868. doi: 10.1152/jn.1987.57.3.835. [DOI] [PubMed] [Google Scholar]
- 44.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 45.Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitboeck HJ. Coherent oscillations: a mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biol Cybern. 1988;60:121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- 46.Eckhorn R. Oscillatory and non-oscillatory synchronizations in the visual cortex and their possible roles in associations of visual features. Prog Brain Res. 1994;102:405–426. doi: 10.1016/S0079-6123(08)60556-7. [DOI] [PubMed] [Google Scholar]
- 47.Eckhorn R. Neural mechanisms of visual feature grouping. Neurol Neurochir Pol. 2000;34:27–42. [PubMed] [Google Scholar]
- 48.Engel AK, Konig P, Gray CM, Singer W. Stimulus-Dependent Neuronal Oscillations in Cat Visual Cortex: Inter-Columnar Interaction as Determined by Cross-Correlation Analysis. Eur J Neurosci. 1990;2:588–606. doi: 10.1111/j.1460-9568.1990.tb00449.x. [DOI] [PubMed] [Google Scholar]
- 49.Engel AK, Konig P, Singer W. Direct physiological evidence for scene segmentation by temporal coding. Proc Natl Acad Sci U S A. 1991;88:9136–9140. doi: 10.1073/pnas.88.20.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci. 2001;5:16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- 51.Ermentrout B, Flores J, Gelperin A. Minimal model of oscillations and waves in the Limax olfactory lobe with tests of the model’s predictive power. J Neurophysiol. 1998;79:2677–2689. doi: 10.1152/jn.1998.79.5.2677. [DOI] [PubMed] [Google Scholar]
- 52.Fahle M. Figure-ground discrimination from temporal information. Proc Biol Sci. 1993;254:199–203. doi: 10.1098/rspb.1993.0146. [DOI] [PubMed] [Google Scholar]
- 53.Fell J, Klaver P, Lehnertz K, Grunwald T, Schaller C, Elger CE, Fernandez G. Human memory formation is accompanied by rhinal-hippocampal coupling and decoupling. Nat Neurosci. 2001;4:1259–1264. doi: 10.1038/nn759. [DOI] [PubMed] [Google Scholar]
- 54.Fell J, Klaver P, Elfadil H, Schaller C, Elger CE, Fernandez G. Rhinal-hippocampal theta coherence during declarative memory formation: interaction with gamma synchronization? Eur J Neurosci. 2003;17:1082–1088. doi: 10.1046/j.1460-9568.2003.02522.x. [DOI] [PubMed] [Google Scholar]
- 55.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 56.Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 57.Freeman WJ. Measurement of oscillatory responses to electrical stimulation in olfactory bulb of cat. J Neurophysiol. 1972;35:762–779. doi: 10.1152/jn.1972.35.6.762. [DOI] [PubMed] [Google Scholar]
- 58.Friedrich RW, Habermann CJ, Laurent G. Multiplexing using synchrony in the zebrafish olfactory bulb. Nat Neurosci. 2004;7:862–871. doi: 10.1038/nn1292. [DOI] [PubMed] [Google Scholar]
- 59.Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 60.Ghose GM, Freeman RD. Oscillatory discharge in the visual system: does it have a functional role? J Neurophysiol. 1992;68:1558–1574. doi: 10.1152/jn.1992.68.5.1558. [DOI] [PubMed] [Google Scholar]
- 61.Ghose GM, Ohzawa I, Freeman RD. Receptive-field maps of correlated discharge between pairs of neurons in the cat’s visual cortex. J Neurophysiol. 1994;71:330–346. doi: 10.1152/jn.1994.71.1.330. [DOI] [PubMed] [Google Scholar]
- 62.Gilbert CD, Wiesel TN. Clustered intrinsic connections in cat visual cortex. J Neurosci. 1983;3:1116–1133. doi: 10.1523/JNEUROSCI.03-05-01116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gray CM, Konig P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- 64.Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci U S A. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gray CM, McCormick DA. Chattering cells: superficial pyramidal neurons contributing to the generation of synchronous oscillations in the visual cortex. Science. 1996;274:109–113. doi: 10.1126/science.274.5284.109. [DOI] [PubMed] [Google Scholar]
- 66.Gray CM, Viana DP. Stimulus-dependent neuronal oscillations and local synchronization in striate cortex of the alert cat. J Neurosci. 1997;17:3239–3253. doi: 10.1523/JNEUROSCI.17-09-03239.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat Rev Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- 68.Gross J, Schmitz F, Schnitzler I, Kessler K, Shapiro K, Hommel B, Schnitzler A. Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proc Natl Acad Sci U S A. 2004;101:13050–13055. doi: 10.1073/pnas.0404944101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grossberg S, Mingolla E, Todorovic D. A neural network architecture for preattentive vision. IEEE Trans Biomed Eng. 1989;36:65–84. doi: 10.1109/10.16450. [DOI] [PubMed] [Google Scholar]
- 70.Grossberg S. Linking the laminar circuits of visual cortex to visual perception: development, grouping, and attention. Neurosci Biobehav Rev. 2001;25:513–526. doi: 10.1016/s0149-7634(01)00030-6. [DOI] [PubMed] [Google Scholar]
- 71.Guderian S, Duzel E. Induced theta oscillations mediate large-scale synchrony with mediotemporal areas during recollection in humans. Hippocampus. 2005;15:901–912. doi: 10.1002/hipo.20125. [DOI] [PubMed] [Google Scholar]
- 72.Hahnloser RH, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature. 2002;419:65–70. doi: 10.1038/nature00974. [DOI] [PubMed] [Google Scholar]
- 73.Hansel D, Sompolinsky H. Chaos and synchrony in a model of a hypercolumn in visual cortex. J Comput Neurosci. 1996;3:7–34. doi: 10.1007/BF00158335. [DOI] [PubMed] [Google Scholar]
- 74.Hebb DO. The Organization of Behavior: a Neuropsychological Theory. Wiley; New York: 1949. [Google Scholar]
- 75.Herrmann CS, Lenz D, Junge S, Busch NA, Maess B. Memory-matches evoke human gamma-responses. BMC Neurosci. 2004;5:13. doi: 10.1186/1471-2202-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hill KG, Stange G, Mo J. Temporal synchronization in the primary auditory response in the pigeon. Hear Res. 1989;39:63–73. doi: 10.1016/0378-5955(89)90082-8. [DOI] [PubMed] [Google Scholar]
- 77.Ikegaya Y, Aaron G, Cossart R, Aronov D, Lampl I, Ferster D, Yuste R. Synfire chains and cortical songs: temporal modules of cortical activity. Science. 2004;304:559–564. doi: 10.1126/science.1093173. [DOI] [PubMed] [Google Scholar]
- 78.Jarvis MR, Mitra PP. Sampling properties of the spectrum and coherency of sequences of action potentials. Neural Comput. 2001;13:717–749. doi: 10.1162/089976601300014312. [DOI] [PubMed] [Google Scholar]
- 79.Jermakowicz WJ, Chen X, Khaytin I, Zhou Z, Bernard MR, Bonds AB, Casagrande VA. Is local neuronal synchrony better at discriminating stimulus spatial frequency in primary visual cortex (V1) than firing rate? Society for Neuroscience Abstracts. 2006 [Google Scholar]
- 80.Jermakowicz WJ, Chen X, Khaytin I, Zhou Z, Bernard MR, Bonds AB, Casagrande VA. Is Synchrony a reasonable coding strategy for visual areas beyond V1 in primates? Vision Sciences Society Abstracts. 2007 [Google Scholar]
- 81.Kara P, Reinagel P, Reid RC. Low response variability in simultaneously recorded retinal, thalamic, and cortical neurons. Neuron. 2000;27:635–646. doi: 10.1016/s0896-6273(00)00072-6. [DOI] [PubMed] [Google Scholar]
- 82.Karbowski J, Kopell N. Multispikes and synchronization in a large neural network with temporal delays. Neural Comput. 2000;12:1573–1606. doi: 10.1162/089976600300015277. [DOI] [PubMed] [Google Scholar]
- 83.Kargo WJ, Nitz DA. Improvements in the signal-to-noise ratio of motor cortex cells distinguish early versus late phases of motor skill learning. J Neurosci. 2004;24:5560–5569. doi: 10.1523/JNEUROSCI.0562-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- 85.Kisvarday ZF, Toth E, Rausch M, Eysel UT. Orientation-specific relationship between populations of excitatory and inhibitory lateral connections in the visual cortex of the cat. Cereb Cortex. 1997;7:605–618. doi: 10.1093/cercor/7.7.605. [DOI] [PubMed] [Google Scholar]
- 86.Kleschevnikov AM, Sokolov MV, Kuhnt U, Dawe GS, Stephenson JD, Voronin LL. Changes in paired-pulse facilitation correlate with induction of long-term potentiation in area CA1 of rat hippocampal slices. Neuroscience. 1997;76:829–843. doi: 10.1016/s0306-4522(96)00342-9. [DOI] [PubMed] [Google Scholar]
- 87.Koch C, Rapp M, Segev I. A brief history of time (constants) Cereb Cortex. 1996;6:93–101. doi: 10.1093/cercor/6.2.93. [DOI] [PubMed] [Google Scholar]
- 88.Koffka K. Principals of Gestalt Psychology. Lund Humphries; London: 1935. [Google Scholar]
- 89.Kohn A, Smith MA. Stimulus dependence of neuronal correlation in primary visual cortex of the macaque. J Neurosci. 2005;25:3661–3673. doi: 10.1523/JNEUROSCI.5106-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Konig P, Engel AK, Singer W. Integrator or coincidence detector? The role of the cortical neuron revisited. Trends Neurosci. 1996;19:130–137. doi: 10.1016/s0166-2236(96)80019-1. [DOI] [PubMed] [Google Scholar]
- 91.Kreiter AK, Singer W. Oscillatory Neuronal Responses in the Visual Cortex of the Awake Macaque Monkey. Eur J Neurosci. 1992;4:369–375. doi: 10.1111/j.1460-9568.1992.tb00884.x. [DOI] [PubMed] [Google Scholar]
- 92.Kreiter AK, Singer W. Stimulus-dependent synchronization of neuronal responses in the visual cortex of the awake macaque monkey. J Neurosci. 1996;16:2381–2396. doi: 10.1523/JNEUROSCI.16-07-02381.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kuba H, Yamada R, Fukui I, Ohmori H. Tonotopic specialization of auditory coincidence detection in nucleus laminaris of the chick. J Neurosci. 2005;25:1924–1934. doi: 10.1523/JNEUROSCI.4428-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuba H, Ishii TM, Ohmori H. Axonal site of spike initiation enhances auditory coincidence detection. Nature. 2006;444:1069–1072. doi: 10.1038/nature05347. [DOI] [PubMed] [Google Scholar]
- 95.Lafreniere-Roula M, McCrea DA. Deletions of rhythmic motoneuron activity during fictive locomotion and scratch provide clues to the organization of the mammalian central pattern generator. J Neurophysiol. 2005;94:1120–1132. doi: 10.1152/jn.00216.2005. [DOI] [PubMed] [Google Scholar]
- 96.Lamme VA, Spekreijse H. Neuronal synchrony does not represent texture segregation. Nature. 1998;396:362–366. doi: 10.1038/24608. [DOI] [PubMed] [Google Scholar]
- 97.Laurent G, Naraghi M. Odorant-induced oscillations in the mushroom bodies of the locust. J Neurosci. 1994;14:2993–3004. doi: 10.1523/JNEUROSCI.14-05-02993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Laurent G. Dynamical representation of odors by oscillating and evolving neural assemblies. Trends Neurosci. 1996;19:489–496. doi: 10.1016/S0166-2236(96)10054-0. [DOI] [PubMed] [Google Scholar]
- 99.Laurent G, Wehr M, Davidowitz H. Temporal representations of odors in an olfactory network. J Neurosci. 1996;16:3837–3847. doi: 10.1523/JNEUROSCI.16-12-03837.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leonards U, Singer W, Fahle M. The influence of temporal phase differences on texture segmentation. Vision Res. 1996;36:2689–2697. doi: 10.1016/0042-6989(96)86829-5. [DOI] [PubMed] [Google Scholar]
- 101.Lowel S, Singer W. Selection of intrinsic horizontal connections in the visual cortex by correlated neuronal activity. Science. 1992;255:209–212. doi: 10.1126/science.1372754. [DOI] [PubMed] [Google Scholar]
- 102.Lumer ED, Edelman GM, Tononi G. Neural dynamics in a model of the thalamocortical system. II. The role of neural synchrony tested through perturbations of spike timing. Cereb Cortex. 1997;7:228–236. doi: 10.1093/cercor/7.3.228. [DOI] [PubMed] [Google Scholar]
- 103.Machens CK, Stemmler MB, Prinz P, Krahe R, Ronacher B, Herz AV. Representation of acoustic communication signals by insect auditory receptor neurons. J Neurosci. 2001;21:3215–3227. doi: 10.1523/JNEUROSCI.21-09-03215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.MacLeod K, Laurent G. Distinct mechanisms for synchronization and temporal patterning of odor-encoding neural assemblies. Science. 1996;274:976–979. doi: 10.1126/science.274.5289.976. [DOI] [PubMed] [Google Scholar]
- 105.MacLeod K, Backer A, Laurent G. Who reads temporal information contained across synchronized and oscillatory spike trains? Nature. 1998;395:693–698. doi: 10.1038/27201. [DOI] [PubMed] [Google Scholar]
- 106.Marois R, Chun MM, Gore JC. Neural correlates of the attentional blink. Neuron. 2000;28:299–308. doi: 10.1016/s0896-6273(00)00104-5. [DOI] [PubMed] [Google Scholar]
- 107.Mason AC, Faure PA. The physiology of insect auditory afferents. Microsc Res Tech. 2004;63:338–350. doi: 10.1002/jemt.20050. [DOI] [PubMed] [Google Scholar]
- 108.Matsuyama K, Nakajima K, Mori F, Aoki M, Mori S. Lumbar commissural interneurons with reticulospinal inputs in the cat: morphology and discharge patterns during fictive locomotion. J Comp Neurol. 2004;474:546–561. doi: 10.1002/cne.20131. [DOI] [PubMed] [Google Scholar]
- 109.Maynard EM, Hatsopoulos NG, Ojakangas CL, Acuna BD, Sanes JN, Normann RA, Donoghue JP. Neuronal interactions improve cortical population coding of movement direction. J Neurosci. 1999;19:8083–8093. doi: 10.1523/JNEUROSCI.19-18-08083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 111.Meijers LM, Eijkman EG. The motor system in simple reaction time experiments. Acta Psychol (Amst) 1974;38:367–377. doi: 10.1016/0001-6918(74)90041-9. [DOI] [PubMed] [Google Scholar]
- 112.Meister M, Lagnado L, Baylor DA. Concerted signaling by retinal ganglion cells. Science. 1995;270:1207–1210. doi: 10.1126/science.270.5239.1207. [DOI] [PubMed] [Google Scholar]
- 113.Melloni L, Molina C, Pena M, Torres D, Singer W, Rodriguez E. Synchronization of neural activity across cortical areas correlates with conscious perception. J Neurosci. 2007;27:2858–2865. doi: 10.1523/JNEUROSCI.4623-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Merriam EB, Netoff TI, Banks MI. Bistable network behavior of layer I interneurons in auditory cortex. J Neurosci. 2005;25:6175–6186. doi: 10.1523/JNEUROSCI.0512-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moiseff A, Konishi M. Neuronal and behavioral sensitivity to binaural time differences in the owl. J Neurosci. 1981;1:40–48. doi: 10.1523/JNEUROSCI.01-01-00040.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Munk MH, Roelfsema PR, Konig P, Engel AK, Singer W. Role of reticular activation in the modulation of intracortical synchronization. Science. 1996;272:271–274. doi: 10.1126/science.272.5259.271. [DOI] [PubMed] [Google Scholar]
- 117.Murthy VN, Fetz EE. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci U S A. 1992;89:5670–5674. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Murthy VN, Fetz EE. Synchronization of neurons during local field potential oscillations in sensorimotor cortex of awake monkeys. J Neurophysiol. 1996;76:3968–3982. doi: 10.1152/jn.1996.76.6.3968. [DOI] [PubMed] [Google Scholar]
- 119.Murthy VN, Fetz EE. Oscillatory activity in sensorimotor cortex of awake monkeys: synchronization of local field potentials and relation to behavior. J Neurophysiol. 1996;76:3949–3967. doi: 10.1152/jn.1996.76.6.3949. [DOI] [PubMed] [Google Scholar]
- 120.Narayanan NS, Kimchi EY, Laubach M. Redundancy and synergy of neuronal ensembles in motor cortex. J Neurosci. 2005;25:4207–4216. doi: 10.1523/JNEUROSCI.4697-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Niebur E, Hsiao SS, Johnson KO. Synchrony: a neuronal mechanism for attentional selection? Curr Opin Neurobiol. 2002;12:190–194. doi: 10.1016/s0959-4388(02)00310-0. [DOI] [PubMed] [Google Scholar]
- 122.Nomura M, Fukai T, Aoyagi T. Synchrony of fast-spiking interneurons interconnected by GABAergic and electrical synapses. Neural Comput. 2003;15:2179–2198. doi: 10.1162/089976603322297340. [DOI] [PubMed] [Google Scholar]
- 123.Nusser Z, Kay LM, Laurent G, Homanics GE, Mody I. Disruption of GABA(A) receptors on GABAergic interneurons leads to increased oscillatory power in the olfactory bulb network. J Neurophysiol. 2001;86:2823–2833. doi: 10.1152/jn.2001.86.6.2823. [DOI] [PubMed] [Google Scholar]
- 124.Oertel D. The role of timing in the brain stem auditory nuclei of vertebrates. Annu Rev Physiol. 1999;61:497–519. doi: 10.1146/annurev.physiol.61.1.497. [DOI] [PubMed] [Google Scholar]
- 125.Optican LM, Richmond BJ. Temporal encoding of two-dimensional patterns by single units in primate inferior temporal cortex. III. Information theoretic analysis. J Neurophysiol. 1987;57:162–178. doi: 10.1152/jn.1987.57.1.162. [DOI] [PubMed] [Google Scholar]
- 126.Palanca BJ, DeAngelis GC. Does neuronal synchrony underlie visual feature grouping? Neuron. 2005;46:333–346. doi: 10.1016/j.neuron.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 127.Palva JM, Palva S, Kaila K. Phase synchrony among neuronal oscillations in the human cortex. J Neurosci. 2005;25:3962–3972. doi: 10.1523/JNEUROSCI.4250-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Paz R, Vaadia E. Learning-induced improvement in encoding and decoding of specific movement directions by neurons in the primary motor cortex. PLoS Biol. 2004;2:E45. doi: 10.1371/journal.pbio.0020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pei X, Vidyasagar TR, Volgushev M, Creutzfeldt OD. Receptive field analysis and orientation selectivity of postsynaptic potentials of simple cells in cat visual cortex. J Neurosci. 1994;14:7130–7140. doi: 10.1523/JNEUROSCI.14-11-07130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- 131.Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, Madsen JR, Lisman JE. Gating of human theta oscillations by a working memory task. J Neurosci. 2001;21:3175–3183. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rall W, Shepherd GM. Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. J Neurophysiol. 1968;31:884–915. doi: 10.1152/jn.1968.31.6.884. [DOI] [PubMed] [Google Scholar]
- 133.Ramachandran VS. Form, motion, and binocular rivalry. Science. 1991;251:950–951. doi: 10.1126/science.2000497. [DOI] [PubMed] [Google Scholar]
- 134.Ramoa AS, Campbell G, Shatz CJ. Dendritic growth and remodeling of cat retinal ganglion cells during fetal and postnatal development. J Neurosci. 1988;8:4239–4261. doi: 10.1523/JNEUROSCI.08-11-04239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Reich DS, Victor JD, Knight BW, Ozaki T, Kaplan E. Response variability and timing precision of neuronal spike trains in vivo. J Neurophysiol. 1997;77:2836–2841. doi: 10.1152/jn.1997.77.5.2836. [DOI] [PubMed] [Google Scholar]
- 136.Reinagel P, Reid RC. Temporal coding of visual information in the thalamus. J Neurosci. 2000;20:5392–5400. doi: 10.1523/JNEUROSCI.20-14-05392.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Richmond BJ, Optican LM. Temporal encoding of two-dimensional patterns by single units in primate inferior temporal cortex. II. Quantification of response waveform. J Neurophysiol. 1987;57:147–161. doi: 10.1152/jn.1987.57.1.147. [DOI] [PubMed] [Google Scholar]
- 138.Richmond BJ, Optican LM, Podell M, Spitzer H. Temporal encoding of two-dimensional patterns by single units in primate inferior temporal cortex. I. Response characteristics. J Neurophysiol. 1987;57:132–146. doi: 10.1152/jn.1987.57.1.132. [DOI] [PubMed] [Google Scholar]
- 139.Rizzuto DS, Madsen JR, Bromfield EB, Schulze-Bonhage A, Seelig D, Aschenbrenner-Scheibe R, Kahana MJ. Reset of human neocortical oscillations during a working memory task. Proc Natl Acad Sci U S A. 2003;100:7931–7936. doi: 10.1073/pnas.0732061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rockland KS, Lund JS. Intrinsic laminar lattice connections in primate visual cortex. J Comp Neurol. 1983;216:303–318. doi: 10.1002/cne.902160307. [DOI] [PubMed] [Google Scholar]
- 141.Rodriguez R, Kallenbach U, Singer W, Munk MH. Short- and long-term effects of cholinergic modulation on gamma oscillations and response synchronization in the visual cortex. J Neurosci. 2004;24:10369–10378. doi: 10.1523/JNEUROSCI.1839-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Roelfsema PR, Lamme VA, Spekreijse H. Synchrony and covariation of firing rates in the primary visual cortex during contour grouping. Nat Neurosci. 2004;7:982–991. doi: 10.1038/nn1304. [DOI] [PubMed] [Google Scholar]
- 143.Rolls ET. Hippocampo-cortical and cortico-cortical backprojections. Hippocampus. 2000;10:380–388. doi: 10.1002/1098-1063(2000)10:4<380::AID-HIPO4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 144.Roy SA, Dear SP, Alloway KD. Long-range cortical synchronization without concomitant oscillations in the somatosensory system of anesthetized cats. J Neurosci. 2001;21:1795–1808. doi: 10.1523/JNEUROSCI.21-05-01795.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Samonds JM, Allison JD, Brown HA, Bonds AB. Cooperation between area 17 neuron pairs enhances fine discrimination of orientation. J Neurosci. 2003;23:2416–2425. doi: 10.1523/JNEUROSCI.23-06-02416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Samonds JM, Bonds AB. From another angle: Differences in cortical coding between fine and coarse discrimination of orientation. J Neurophysiol. 2004;91:1193–1202. doi: 10.1152/jn.00829.2003. [DOI] [PubMed] [Google Scholar]
- 147.Samonds JM, Allison JD, Brown HA, Bonds AB. Cooperative synchronized assemblies enhance orientation discrimination. Proc Natl Acad Sci U S A. 2004;101:6722–6727. doi: 10.1073/pnas.0401661101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Samonds JM, Bonds AB. From another angle: Differences in cortical coding between fine and coarse discrimination of orientation. J Neurophysiol. 2004;91:1193–1202. doi: 10.1152/jn.00829.2003. [DOI] [PubMed] [Google Scholar]
- 149.Samonds JM, Bonds AB. Gamma oscillation maintains stimulus structure-dependent synchronization in cat visual cortex. J Neurophysiol. 2005;93:223–236. doi: 10.1152/jn.00548.2004. [DOI] [PubMed] [Google Scholar]
- 150.Samonds JM, Zhou Z, Bernard MR, Bonds AB. Synchronous activity in cat visual cortex encodes collinear and cocircular contours. J Neurophysiol. 2006;95:2602–2616. doi: 10.1152/jn.01070.2005. [DOI] [PubMed] [Google Scholar]
- 151.Schomburg ED, Petersen N, Barajon I, Hultborn H. Flexor reflex afferents reset the step cycle during fictive locomotion in the cat. Exp Brain Res. 1998;122:339–350. doi: 10.1007/s002210050522. [DOI] [PubMed] [Google Scholar]
- 152.Schuster HG, Wagner P. A model for neuronal oscillations in the visual cortex. 2. Phase description of the feature dependent synchronization. Biol Cybern. 1990;64:83–85. doi: 10.1007/BF00203634. [DOI] [PubMed] [Google Scholar]
- 153.Schuster HG, Wagner P. A model for neuronal oscillations in the visual cortex. 1. Mean-field theory and derivation of the phase equations. Biol Cybern. 1990;64:77–82. doi: 10.1007/BF00203633. [DOI] [PubMed] [Google Scholar]
- 154.Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci. 2003;23:10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shadlen MN, Movshon JA. Synchrony unbound: a critical evaluation of the temporal binding hypothesis. Neuron. 1999;24:67–25. doi: 10.1016/s0896-6273(00)80822-3. [DOI] [PubMed] [Google Scholar]
- 156.Sheinberg DL, Logothetis NK. The role of temporal cortical areas in perceptual organization. Proc Natl Acad Sci U S A. 1997;94:3408–3413. doi: 10.1073/pnas.94.7.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sherrington CS. Observations on the scratch-reflex in the spinal dog. J Physiol. 1906;34:1–50. doi: 10.1113/jphysiol.1906.sp001139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sherrington CS. Man on His Nature. Cambridge University Press; London: 1941. [Google Scholar]
- 159.Shimojo S, Silverman GH, Nakayama K. An occlusion-related mechanism of depth perception based on motion and interocular sequence. Nature. 1988;333:265–268. doi: 10.1038/333265a0. [DOI] [PubMed] [Google Scholar]
- 160.Sillito AM, Jones HE, Gerstein GL, West DC. Feature-linked synchronization of thalamic relay cell firing induced by feedback from the visual cortex. Nature. 1994;369:479–482. doi: 10.1038/369479a0. [DOI] [PubMed] [Google Scholar]
- 161.Singer W, Lux HD. Presynaptic depolarization and extracellular potassium in the cat lateral geniculate nucleus. Brain Res. 1973;64:17–33. doi: 10.1016/0006-8993(73)90168-6. [DOI] [PubMed] [Google Scholar]
- 162.Singer W, Bedworth N. Inhibitory interaction between X and Y units in the cat lateral geniculate nucleus. Brain Res. 1973;49:291–307. doi: 10.1016/0006-8993(73)90424-1. [DOI] [PubMed] [Google Scholar]
- 163.Singer W. Synchronization of cortical activity and its putative role in information processing and learning. Annu Rev Physiol. 1993;55:349–374. doi: 10.1146/annurev.ph.55.030193.002025. [DOI] [PubMed] [Google Scholar]
- 164.Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- 165.Smith AT, Harris LR. Use of plaid patterns to distinguish the corticofugal and direct retinal inputs to the brainstem optokinetic nystagmus generator. Exp Brain Res. 1991;86:324–332. doi: 10.1007/BF00228955. [DOI] [PubMed] [Google Scholar]
- 166.Softky W. Sub-millisecond coincidence detection in active dendritic trees. Neuroscience. 1994;58:13–41. doi: 10.1016/0306-4522(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 167.Srinivasan R, Russell DP, Edelman GM, Tononi G. Increased synchronization of neuromagnetic responses during conscious perception. J Neurosci. 1999;19:5435–5448. doi: 10.1523/JNEUROSCI.19-13-05435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Steinmetz PN, Roy A, Fitzgerald PJ, Hsiao SS, Johnson KO, Niebur E. Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature. 2000;404:187–190. doi: 10.1038/35004588. [DOI] [PubMed] [Google Scholar]
- 169.Stellwagen D, Shatz CJ. An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron. 2002;33:357–367. doi: 10.1016/s0896-6273(02)00577-9. [DOI] [PubMed] [Google Scholar]
- 170.Stoner GR, Albright TD, Ramachandran VS. Transparency and coherence in human motion perception. Nature. 1990;344:153–155. doi: 10.1038/344153a0. [DOI] [PubMed] [Google Scholar]
- 171.Stoner GR, Albright TD. The interpretation of visual motion: evidence for surface segmentation mechanisms. Vision Res. 1996;36:1291–1310. doi: 10.1016/0042-6989(95)00195-6. [DOI] [PubMed] [Google Scholar]
- 172.Stopfer M, Bhagavan S, Smith BH, Laurent G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390:70–74. doi: 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- 173.Stopfer M, Laurent G. Short-term memory in olfactory network dynamics. Nature. 1999;402:664–668. doi: 10.1038/45244. [DOI] [PubMed] [Google Scholar]
- 174.Struber D, Basar-Eroglu C, Hoff E, Stadler M. Reversal-rate dependent differences in the EEG gamma-band during multistable visual perception. Int J Psychophysiol. 2000;38:243–252. doi: 10.1016/s0167-8760(00)00168-9. [DOI] [PubMed] [Google Scholar]
- 175.Tallon-Baudry C, Bertrand O, Delpuech C, Permier J. Oscillatory gamma-band (30–70 Hz) activity induced by a visual search task in humans. J Neurosci. 1997;17:722–734. doi: 10.1523/JNEUROSCI.17-02-00722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- 177.Tallon-Baudry C, Bertrand O, Fischer C. Oscillatory synchrony between human extrastriate areas during visual short-term memory maintenance. J Neurosci. 2001;21:RC177. doi: 10.1523/JNEUROSCI.21-20-j0008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tallon-Baudry C, Mandon S, Freiwald WA, Kreiter AK. Oscillatory synchrony in the monkey temporal lobe correlates with performance in a visual short-term memory task. Cereb Cortex. 2004;14:713–720. doi: 10.1093/cercor/bhh031. [DOI] [PubMed] [Google Scholar]
- 179.Tamas G, Buhl EH, Lorincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000;3:366–371. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- 180.Taylor K, Mandon S, Freiwald WA, Kreiter AK. Coherent oscillatory activity in monkey area v4 predicts successful allocation of attention. Cereb Cortex. 2005;15:1424–1437. doi: 10.1093/cercor/bhi023. [DOI] [PubMed] [Google Scholar]
- 181.Thiele A, Stoner G. Neuronal synchrony does not correlate with motion coherence in cortical area MT. Nature. 2003;421:366–370. doi: 10.1038/nature01285. [DOI] [PubMed] [Google Scholar]
- 182.Tiesinga PH, Fellous JM, Salinas E, Jose JV, Sejnowski TJ. Inhibitory synchrony as a mechanism for attentional gain modulation. J Physiol Paris. 2004;98:296–314. doi: 10.1016/j.jphysparis.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tomita M, Eggermont JJ. Cross-correlation and joint spectro-temporal receptive field properties in auditory cortex. J Neurophysiol. 2005;93:378–392. doi: 10.1152/jn.00643.2004. [DOI] [PubMed] [Google Scholar]
- 184.Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- 185.Usrey WM, Reid RC. Synchronous activity in the visual system. Annu Rev Physiol. 1999;61:435–456. doi: 10.1146/annurev.physiol.61.1.435. [DOI] [PubMed] [Google Scholar]
- 186.Usrey WM, Alonso JM, Reid RC. Synaptic interactions between thalamic inputs to simple cells in cat visual cortex. J Neurosci. 2000;20:5461–5467. doi: 10.1523/JNEUROSCI.20-14-05461.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.van der TC, Kalitzin S, Spekreijse H, Lamme VA, Super H. Synchrony dynamics in monkey V1 predict success in visual detection. Cereb Cortex. 2006;16:136–148. doi: 10.1093/cercor/bhi093. [DOI] [PubMed] [Google Scholar]
- 188.van Vreeswijk C, Sompolinsky H. Chaos in neuronal networks with balanced excitatory and inhibitory activity. Science. 1996;274:1724–1726. doi: 10.1126/science.274.5293.1724. [DOI] [PubMed] [Google Scholar]
- 189.Vidal JR, Chaumon M, O’Regan JK, Tallon-Baudry C. Visual grouping and the focusing of attention induce gamma-band oscillations at different frequencies in human magnetoencephalogram signals. J Cogn Neurosci. 2006;18:1850–1862. doi: 10.1162/jocn.2006.18.11.1850. [DOI] [PubMed] [Google Scholar]
- 190.Vingerhoets G. Cognitive effects of seizures. Seizure. 2006 doi: 10.1016/j.seizure.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 191.Vislay-Meltzer RL, Kampff AR, Engert F. Spatiotemporal specificity of neuronal activity directs the modification of receptive fields in the developing retinotectal system. Neuron. 2006;50:101–114. doi: 10.1016/j.neuron.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 192.Volgushev M, Voronin LL, Chistiakova M, Singer W. Relations between long-term synaptic modifications and paired-pulse interactions in the rat neocortex. Eur J Neurosci. 1997;9:1656–1665. doi: 10.1111/j.1460-9568.1997.tb01523.x. [DOI] [PubMed] [Google Scholar]
- 193.von der MC, Cowan JD. Outline of a theory for the ontogenesis of iso-orientation domains in visual cortex. Biol Cybern. 1982;45:49–56. doi: 10.1007/BF00387213. [DOI] [PubMed] [Google Scholar]
- 194.Voronin LL, Volgushev M, Chistiakova M, Kuhnt U, Singer W. Involvement of silent synapses in the induction of long-term potentiation and long-term depression in neocortical and hippocampal neurons. Neuroscience. 1996;74:323–330. doi: 10.1016/0306-4522(96)00207-2. [DOI] [PubMed] [Google Scholar]
- 195.Ward LM. Synchronous neural oscillations and cognitive processes. Trends Cogn Sci. 2003;7:553–559. doi: 10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 196.Wehr M, Laurent G. Odour encoding by temporal sequences of firing in oscillating neural assemblies. Nature. 1996;384:162–166. doi: 10.1038/384162a0. [DOI] [PubMed] [Google Scholar]
- 197.Westheimer G. Cooperative neural processes involved in stereoscopic acuity. Exp Brain Res. 1979;36:585–597. doi: 10.1007/BF00238525. [DOI] [PubMed] [Google Scholar]
- 198.Wiencken AE, Casagrande VA. Endothelial nitric oxide synthetase (eNOS) in astrocytes: another source of nitric oxide in neocortex. Glia. 1999;26:280–290. [PubMed] [Google Scholar]
- 199.Womelsdorf T, Fries P, Mitra PP, Desimone R. Gamma-band synchronization in visual cortex predicts speed of change detection. Nature. 2006;439:733–736. doi: 10.1038/nature04258. [DOI] [PubMed] [Google Scholar]
- 200.Woolley SM, Gill PR, Theunissen FE. Stimulus-dependent auditory tuning results in synchronous population coding of vocalizations in the songbird midbrain. J Neurosci. 2006;26:2499–2512. doi: 10.1523/JNEUROSCI.3731-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yin TC, Kuwada S. Binaural interaction in low-frequency neurons in inferior colliculus of the cat. II. Effects of changing rate and direction of interaural phase. J Neurophysiol. 1983;50:1000–1019. doi: 10.1152/jn.1983.50.4.1000. [DOI] [PubMed] [Google Scholar]
- 202.Young MP, Tanaka K, Yamane S. On oscillating neuronal responses in the visual cortex of the monkey. J Neurophysiol. 1992;67:1464–1474. doi: 10.1152/jn.1992.67.6.1464. [DOI] [PubMed] [Google Scholar]
- 203.Zochowski MR, Cohen LB. Oscillations in the olfactory bulb carry information about odorant history. J Neurophysiol. 2005;94:2667–2675. doi: 10.1152/jn.00328.2005. [DOI] [PubMed] [Google Scholar]
- 204.Zohary E, Shadlen MN, Newsome WT. Correlated neuronal discharge rate and its implications for psychophysical performance. Nature. 1994;370:140–143. doi: 10.1038/370140a0. [DOI] [PubMed] [Google Scholar]