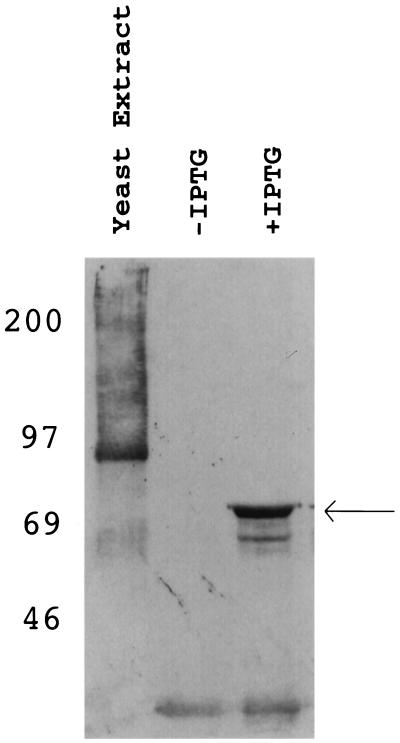
Figure 4.
Protein encoded by the CML33 cDNA cross-reacts with antibodies against sheep PheRS. Total protein from S. cerevisiae (lane 1) or total protein from E. coli containing a gene encoding the glutathione-S-transferase–CML33 fusion protein, uninduced (lane 2) and induced (lane 3) with IPTG, were immunoblotted with antibody against sheep PheRS. The cross-reacting material in the yeast extract lane does not correspond to any subunit of yeast cytoplasmic (approximate molecular weights of 70,000 and 60,000) or mitochondrial (molecular weight of about 57,000) PheRS. Thus, the antibodies are sufficiently specific to distinguish between yeast and mammalian PheRS. Note the fusion protein band in lane 3, marked with an arrow. A molecular weight of 82,000 is predicted for the fusion protein, which is close to what is observed.