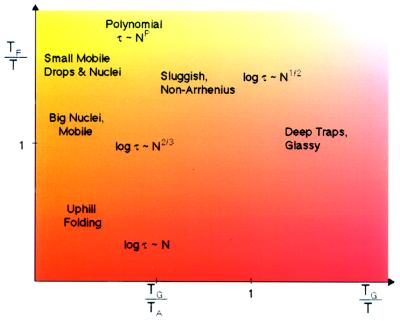
Figure 3.
The various regimes of scaling behavior for the typical folding time with length are indicated for different values of TF/T, the folding temperature-to-ambient ratio, and TG/T, the glass temperature-to-ambient ratio. At high temperatures, folding is always thermodynamic uphill and the barrier grows like N. At low temperatures, the rate is trap-dominated, and the typical barrier behaves like N1/2, whereas the slowest escape (not indicated in the figure) will have bigger barriers, scaling like N. Fastest folding occurs for T ≪ TF but T ≫ TG where polynomial behavior of the folding time in N is to be found. This occurs for “well designed” proteins. The figure also shows the boundary between trap escape and quasi-free chain dynamics, as indicated by the mean field dynamic transition temperature TA. A realistic value of TF/TG is 1.6. In this figure we assume temperatures are all less than the collapse temperature TC. If T is bigger than TC, ruggedness effects can be neglected.