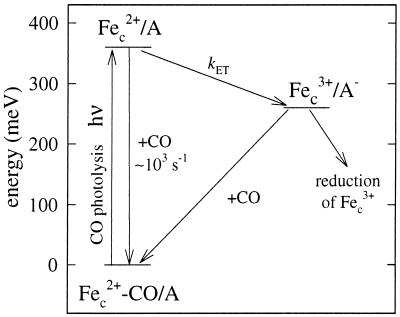
Figure 5.
Energy-level diagram. After mixing of CmCyt c-CO (Fec2+-CO) and the electron acceptor (A) a complex is formed. Flash photolysis of CO results in a drop of the redox potential (increase in energy) by about 360 mV at 1 mM CO. The CO-recombination rate is ≈103 s−1 (at 1 mM CO). After dissociation of CO, electron transfer from CmCyt c to A proceeds with a rate constant kET. This is followed by slow re-reduction of CmCyt c by A− and CO recombination. Alternatively, if a reductant is present in solution (as in the experiments in this work), it may re-reduce CmCyt c directly.