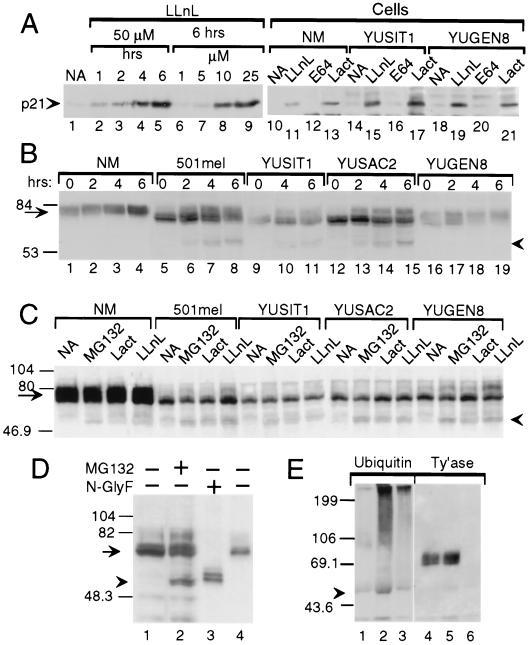
Figure 4.
Evidence for tyrosinase degradation by the proteasome–ubiquitin pathway. Western blots with anti-p21 (A), anti-tyrosinase (polyclonal, B; monoclonal, C–E) or anti-ubiquitin (E) antibodies. (A) Proteasome inhibitors block p21WAF1/Cip1 degradation. Accumulation of p21 in response to LLnL in metastatic melanoma line YUPAC7 as a function of time and concentration (lanes 1–9) and in normal melanocytes (NM) and melanoma cell lines after treatment for 4 h with LLnL (50 μM) or with lactacystin (Lact, 60 μM) but not with DMSO (0.1%, NA) or E64 (50 μM) (lanes 10–21). (B) Accumulation of 60-kDa tyrosinase doublet and increased efficiency of tyrosinase processing in response to LLnL. Shown is tyrosinase in whole cell lysates (40 μg/lane) from normal melanocytes (NM) or melanoma cells treated with DMSO for 6 h (lanes marked 0) or LLnL (50 μM) for 2, 4, or 6 h. (C) Accumulation of 60-kDa tyrosinase doublet by additional inhibitors. Cells were treated for 3 h with DMSO (NA), MG132 (50 μM), lactacystin (Lact, 30 μM), or LLnL (50 μM). (D) The 60-kDa band comigrates with deglycosylated tyrosinase. Lanes: 1 and 2, tyrosinase in whole cell lysates from 501mel cells treated for 6 h with DMSO (−) or MG132 (+, 50 μM); 3 and 4, lactin-bound glycoproteins from 501mel cells after overnight incubation without (−) or with N-glycosidase F (N-glyF, +). (E) Ubiquitination of tyrosinase. Products of immunoprecipitations with anti-tyrosinase polyclonal antibodies (lanes 1, 2, 4, and 5) or control rabbit IgG (lanes 3 and 6) were Western blotted with anti-ubiquitin 4F3 mAb. After stripping, the same membrane was probed with anti-tyrosinase mAb (Ty’ase). Extracts were from normal melanocytes incubated for 4 h with DMSO (lanes 1 and 4) or LLnL (50 μM, lanes 2, 3, 5, and 6). Arrowheads in B–E point at the 60-kDa doublet of tyrosinase. Arrows point at normal and melanoma tyrosinase glycoproteins.