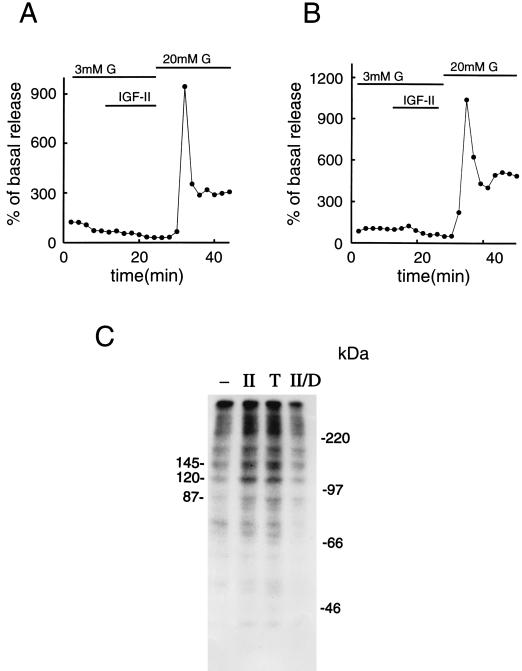
Figure 4.
Role of GTP binding proteins, PKC, and phosphorylation in mediating the effect of IGF-II on insulin release. (A) β cells were incubated in RPMI medium 1640 supplemented with 50 ng/ml pertussis toxin for 8 hr. Thereafter, they were subjected to column perifusion and stimulation with 50 ng/ml IGF-II for 14 min at a basal glucose concentration of 3 mM. (B) A similar type of experiment was performed with β cells incubated in RPMI medium 1640 supplemented with 1 μM TPA for 3 hr, to down-regulate PKC activity. In both A and B, the cells were exposed to 20 mM glucose at the end of the experiment to ensure that they were still capable of secreting normal amounts of the hormone. Insulin release is expressed as percentage of basal secretion, obtained at 3 mM glucose. Representative experiments out of three are shown. (C) Protein phosphorylation in β cells incubated for 4 min at 37°C in buffer only (−), in the presence of 50 ng/ml IGF-II (II), 25 nM TPA (T), or 50 ng/ml IGF-II, subsequent to down-regulation of PKC activity (II/D). Three prominent protein bands and protein molecular mass standards are indicated (in kilodaltons). In C, a representative experiment out of three is shown.