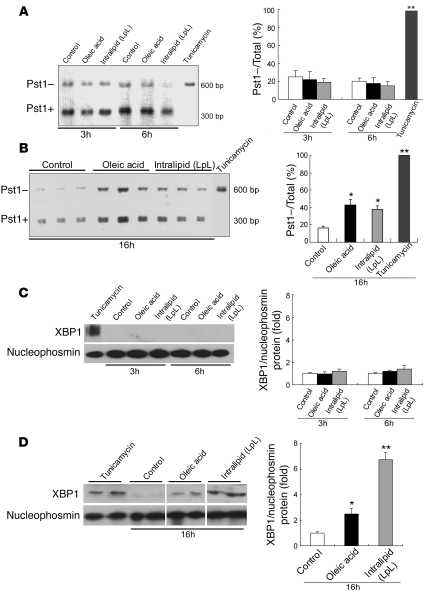
Figure 4. OA or IL induces alternative splicing of XBP1 mRNA and translocation of the alternative form of the protein into the nucleus in McA cells.
McA cells were exposed for 3 or 6 hours (A) or for 16 hours (B) to OA (0.4 mM) or IL (500 mg/dl). XBP1 cDNA was amplified by PCR followed by incubation with Pst1. The products of the incubation with Pst1 are shown on the left; the bar graphs show the percentage of total XBP1 mRNA that was resistant to Pst1 and was, therefore, already spliced and activated. After 3 or 6 hours of incubation with OA or IL, most of the XBP1 PCR products were cut by Pst1 (Pst1+), producing a 300-bp amplification product, indicating a predominance of the native, unspliced form of XBP1 mRNA; less than 20% of total XBP1 was detected as the Pst1–, 601-bp amplification product, indicative of spliced XBP1 mRNA. By contrast, after a 16-hour incubation with either OA or IL, a larger proportion of the XBP1 PCR product was Pst1– and kept its full 601-bp length (OA 43% ± 6%; IL 38% ± 4%), indicating partial XBP1 mRNA splicing and presence of ER stress. Complete XBP1 activation and splicing were induced by a 3-hour treatment with tunicamycin (5 μg/ml) as a positive control. Data are mean ± SD (n = 6 for each condition); *P < 0.05, **P < 0.01 versus control incubations (without OA or IL). The nuclear content of XBP1 was not increased after a 6-hour incubation with OA or IL (C) but was increased after incubation with either lipid source for 16 hours (D). Nucleophosmin is shown as a control for the efficiency of the nuclear extraction. ER stress was also induced by a 3-hour treatment with 5 μg/ml tunicamycin as a positive control. Lanes were run on the same gel but were noncontiguous. Data are mean ± SD normalized to cells incubated without OA or IL (n = 3 for each condition); *P < 0.05, **P < 0.01 versus control incubations.