Figure 6.
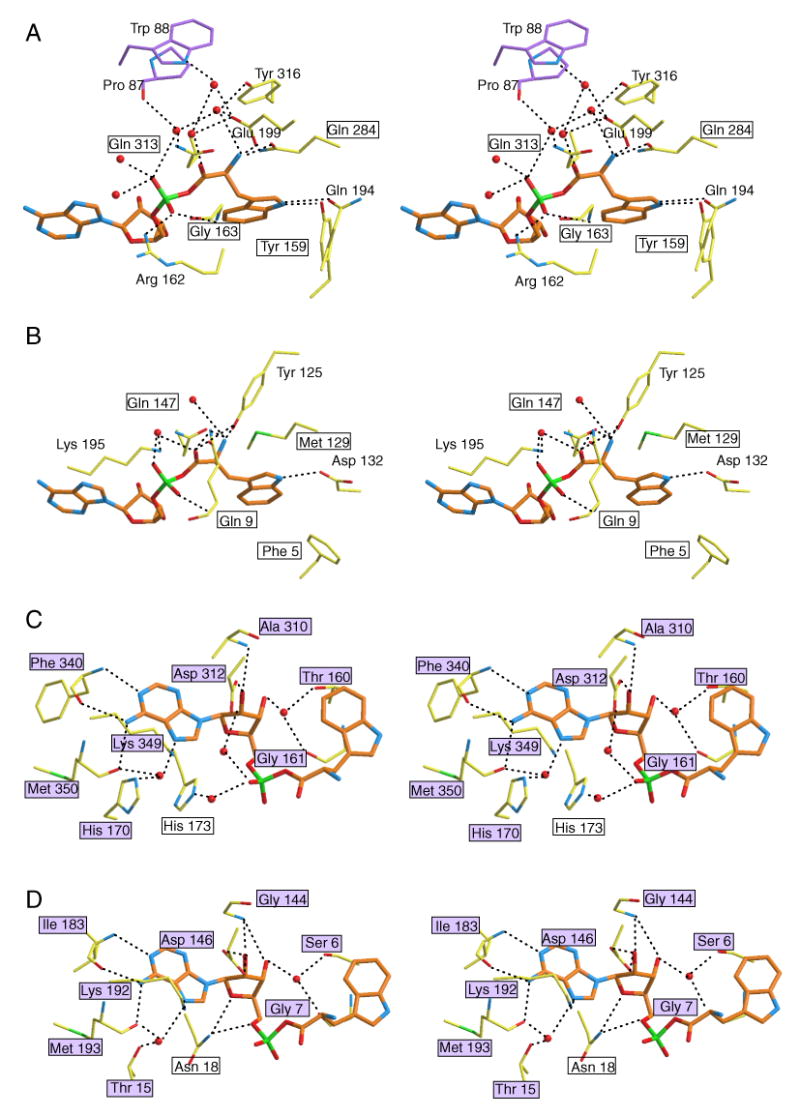
Close-up views of the Trp-AMP binding site of human (A, C) and B. stearothermophilus (B, D) TrpRS. Equivalent residues between the two TrpRSs are boxed, and the purple color-filled boxes indicate conserved protein-ligand interactions. Clearly, interactions with the Trp moiety are less conserved between human TrpRS (A) and B. stearothermophilus TrpRS (B), compared to interactions with the AMP moiety (C, D). (To reduce overlap and increase clarity for panel C and D, some interactions with the α-phosphate group are illustrated in panel A and B.)
