Figure 7.
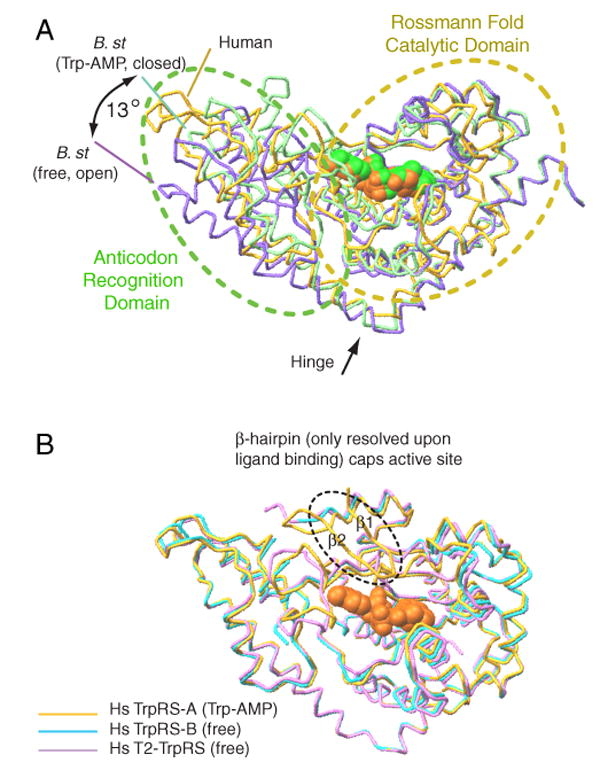
Human and B. stearothermophilus TrpRSs use distinct mechanism to close the active site for catalysis. (A) In B. stearothermophilus TrpRS, ligand binding induces a 13° ‘hinge-like’ motion— between the Rossmann fold domain and the anticodon recognition domain—to close the ATP binding cleft. (B) Human TrpRS stays in a conformation similar to the ‘closed’ conformation of B. stearothermophilus TrpRS. Ligand binding engages the β1-β2 hairpin (from the eukaryote-specific patch) to cap the active site.
