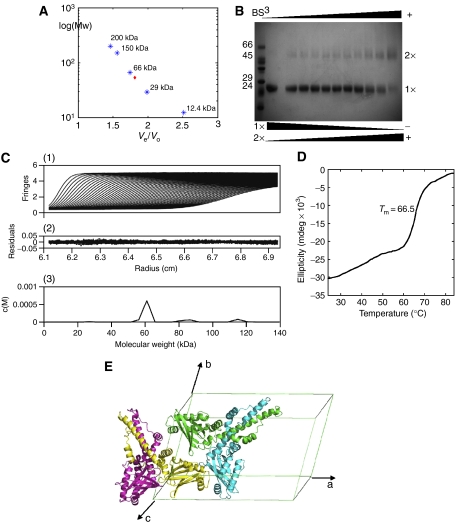
Figure 3.
Evidence for the XLF dimer. (A) Superdex-200 calibration curves were used to estimate the molecular weight of the XLF multimer. Proteins used for calibration are shown in blue stars, and the red diamond indicates XLF elution. (B) Crosslinking with BS3 indicated the existence of an XLF dimer, the amount of which was enhanced by increasing BS3, while the monomer decreased at the same time. (C) Sedimentation velocity profiles of XLF (1.8 mg/ml) centrifuged at 20°C and a rotor speed of 55 000 r.p.m. (1) and the residuals obtained after data fitting (2). The peak at 60 kDa (3) corresponds to the XLF dimer, which takes a relevant concentration of 92%. Data were analysed using SEDFIT program (Schuck, 2000). (D) Thermal denaturation experiment performed by CD. Tm is measured as 66.5°C. (E) XLF forms a homodimer with a two-fold axis relating protomers, and two XLF homodimers are packed in one asymmetric unit of the C2 cell.