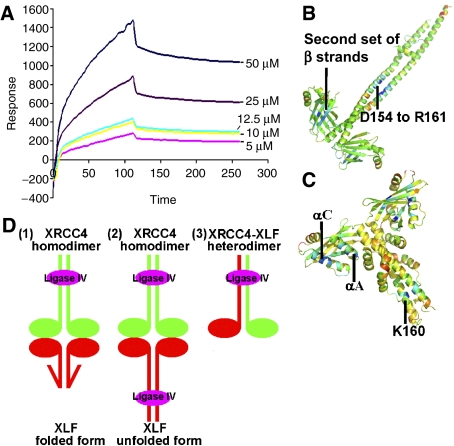
Figure 6.
(A) XLF–XRCC4 interactions evaluated by BIAcore 2000. Sensorgrams obtained from the injections of XRCC4 over the immobilised XLF surface at concentrations of 50, 25, 12.5, 10, 5 μM. (B, C) Prediction of regions that will favour protein–protein interactions in XRCC4 and XLF structures. The darker the blue colour of a region of the dimer, the greater the probability that it acts as a binding region as indicated by ODA (Fernandez-Recio et al, 2005). XRCC4 probably interacts with other molecules through the head domains and the coiled-coil region. XLF head domains are likely to interact with other factors but also a region surrounding the conserved, K160 residue, is highlighted. (D) Possible modes of interaction between XRCC4, XLF and Ligase IV. XRCC4 molecules are shown in green, XLF is in red and Ligase IV BRCR-linker region is in magenta. (1) Linker region between Ligase IV BRCT domains binds to XRCC4's coiled-coil, folded XLF/Cernunnos contacts XRCC4 via the head domains. (2) The C termini of XLF molecules are unfolded and bind to Ligase IV in a similar way to XRCC4. Thus, there are two Ligase IV molecules in this large complex. (3) XLF and XRCC4 form a heterodimer and bind to Ligase IV in the composite coiled-coil region.