FIGURE 5. Dominant positive arrestin rescues morphine-mediated and GRK-mediated activation of phospho-ERK1/2.
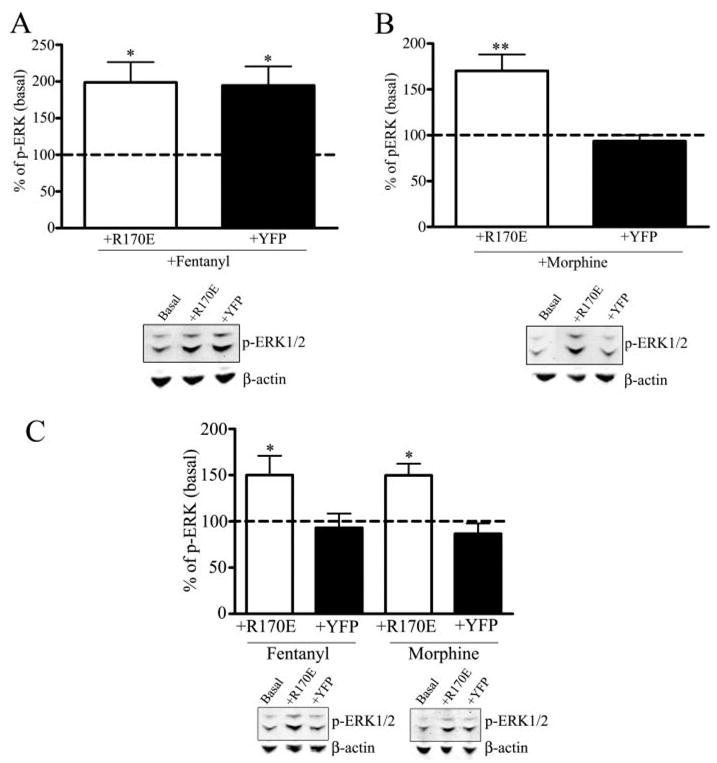
Morphine-induced activation of phospho-ERK1/2 by transfection of the dominant positive arrestin R170E in wild type and GRK3−/− striatal neurons. A, upper panel, striatal cultures were transfected with the dominant positive arrestin3-(R170E)-YFP or empty vector YFP and treated with 100 nM fentanyl. The intensity of the phospho-ERK1/2 immunoreactivity in agonist-treated transfected neurons was compared with untreated striatal neurons transfected with the dominant positive arrestin3-(R170E)-YFP (Basal). Fentanyl treatment increased the activation of phospho-ERK1/2 compared with basal levels (*, p < 0.05 by Dunnett’s post hoc comparison, n = 4). Fentanyl-treated YFP-transfected neurons increased the activation of phospho-ERK1/2 compared with basal levels (*, p < 0.05 by Dunnett’s post hoc comparison, n = 4). A, lower panel, a representative immunoblot for the 44/42-kDa phospho-ERK1/2 protein is shown in which cells were untreated in the presence of the dominant positive arrestin-(R170E) (Basal), treated with fentanyl in the presence of the dominant positive arrestin-(R170E) (+R170E) or YFP (+YFP). Representative immunoblots for β-actin demonstrates equal protein loading for each representative experiment. B, upper panel, striatal cultures were transfected with the dominant positive arrestin3-(R170E)-YFP or empty vector YFP and treated with 10 μM morphine for 30 min. The intensity of the phospho-ERK1/2 immunoreactivity in agonist-treated transfected neurons was compared with untreated striatal neurons transfected with the dominant positive arrestin3-(R170E)-YFP (Basal). Morphine treatment increased the activation of phospho-ERK1/2 compared with basal levels (**, p < 0.01 by Dunnett’s post hoc comparison, n = 4). B, lower panel, a representative immunoblot for the 44/42-kDa phospho-ERK1/2 protein is shown in which cells were untreated in the presence of the dominant positive arrestin-(R170E) (Basal), treated with morphine in the presence of the dominant positive arrestin-(R170E) (+R170E) or YFP (+YFP). Representative immunoblots for β-actin demonstrates equal protein loading for each representative experiment. C, GRK3−/− striatal cultures were transfected with the dominant positive arrestin3-(R170E)-YFP or empty vector YFP and treated with 100 nM fentanyl or 10 μM morphine for 30 min. The intensity of the phospho-ERK1/2 immunoreactivity in agonist-treated transfected neurons was compared with untreated striatal neurons transfected with the dominant positive arrestin3-(R170E)-YFP (basal). Fentanyl treatment increased the activation of phospho-ERK1/2 compared with basal levels (*, p < 0.05 by Dunnett’s post hoc comparison, n = 4). Morphine treatment increased the activation of phospho-ERK1/2 compared with basal levels (*, p < 0.05 by Dunnett’s post hoc comparison, n = 4). C, lower panel, a representative immunoblot for the 44/42-kDa phospho-ERK1/2 protein is shown in which cells were untreated in the presence of the dominant positive arrestin-(R170E) (Basal), treated with fentanyl in the presence of the dominant positive arrestin R170E (+R170E) or YFP (+YFP). Representative immunoblots for β-actin demonstrates equal protein loading for each representative experiment. C, lower panel, a representative immunoblot for the 44/42-kDa phospho-ERK1/2 protein is shown in which cells were untreated in the presence of the dominant positive arrestin-(R170E) (Basal), treated with morphine in the presence of the dominant positive arrestin-(R170E) (+R170E) or YFP (+YFP). Representative immunoblots for β-actin demonstrates equal protein loading for each representative experiment.
