Abstract
Mutant forms of elongation factor Tu encoded by tufA8 and tufB103 in Salmonella typhimurium cause suppression of some but not all frameshift mutations. All of the suppressed mutations in S. typhimurium have frameshift windows ending in the termination codon UGA. Because both tufA8 and tufB103 are moderately efficient UGA suppressors, we asked whether the efficiency of frameshifting is influenced by the level of misreading at UGA. We introduced plasmids synthesizing either one of the release factors into strains in which the tuf mutations suppress a test frameshift mutation. We found that overproduction of release factor 2 (which catalyzes release at UGA and UAA) reduced frameshifting promoted by the tuf mutations at all sites tested. However, at one of these sites, trpE91, overproduction of release factor 1 also reduced suppression. The spontaneous level of frameshift "leakiness" at three sites in trpE, each terminating in UGA, was reduced in strains carrying the release factor 2 plasmid. We conclude that both spontaneous and suppressor-enhanced reading-frame shifts are influenced by the activity of peptide chain release factors. However, the data suggest that the effect of release factor on frameshifting does not necessarily depend on the presence of the normal triplet termination signal.
Full text
PDF


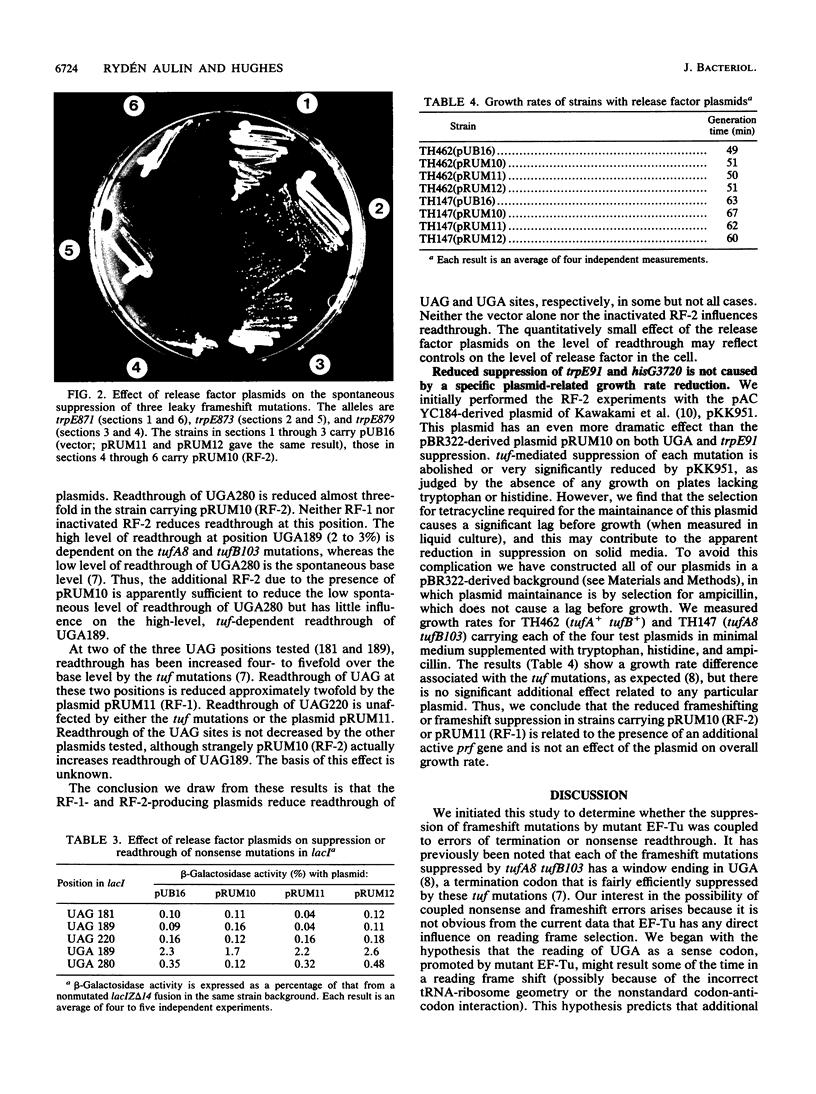


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins J. F., Nichols B. P., Thompson S. The nucleotide sequence of the first externally suppressible--1 frameshift mutant, and of some nearby leaky frameshift mutants. EMBO J. 1983;2(8):1345–1350. doi: 10.1002/j.1460-2075.1983.tb01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins J. F., Ryce S. UGA and non-triplet suppressor reading of the genetic code. Nature. 1974 Jun 7;249(457):527–530. doi: 10.1038/249527a0. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Stockwell P. A., Trotman C. N., Tate W. P. The signal for the termination of protein synthesis in procaryotes. Nucleic Acids Res. 1990 Apr 25;18(8):2079–2086. doi: 10.1093/nar/18.8.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R., Klein H. A. Characterization of three proteins involved in polypeptide chain termination. Cold Spring Harb Symp Quant Biol. 1969;34:469–477. doi: 10.1101/sqb.1969.034.01.053. [DOI] [PubMed] [Google Scholar]
- Hughes D., Atkins J. F., Thompson S. Mutants of elongation factor Tu promote ribosomal frameshifting and nonsense readthrough. EMBO J. 1987 Dec 20;6(13):4235–4239. doi: 10.1002/j.1460-2075.1987.tb02772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes D. Mutant forms of tufA and tufB independently suppress nonsense mutations. J Mol Biol. 1987 Oct 20;197(4):611–615. doi: 10.1016/0022-2836(87)90467-0. [DOI] [PubMed] [Google Scholar]
- Kawakami K., Jönsson Y. H., Björk G. R., Ikeda H., Nakamura Y. Chromosomal location and structure of the operon encoding peptide-chain-release factor 2 of Escherichia coli. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5620–5624. doi: 10.1073/pnas.85.15.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R., Hearn M., Jenny P., Gallant J. Release factor competition is equivalent at strong and weakly suppressed nonsense codons. Mol Gen Genet. 1988 Jul;213(1):144–149. doi: 10.1007/BF00333411. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Coulondre C., Farabaugh P. J. Correlation of nonsense sites in the lacI gene with specific codons in the nucleotide sequence. Nature. 1978 Aug 24;274(5673):770–775. doi: 10.1038/274770a0. [DOI] [PubMed] [Google Scholar]
- Murgola E. J., Hijazi K. A., Göringer H. U., Dahlberg A. E. Mutant 16S ribosomal RNA: a codon-specific translational suppressor. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4162–4165. doi: 10.1073/pnas.85.12.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Hill B., Kania J. Lac repressor can be fused to beta-galactosidase. Nature. 1974 Jun 7;249(457):561–563. doi: 10.1038/249561a0. [DOI] [PubMed] [Google Scholar]
- O'Connor M., Gesteland R. F., Atkins J. F. tRNA hopping: enhancement by an expanded anticodon. EMBO J. 1989 Dec 20;8(13):4315–4323. doi: 10.1002/j.1460-2075.1989.tb08618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony D. J., Hughes D., Thompson S., Atkins J. F. Suppression of a -1 frameshift mutation by a recessive tRNA suppressor which causes doublet decoding. J Bacteriol. 1989 Jul;171(7):3824–3830. doi: 10.1128/jb.171.7.3824-3830.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R. E. The nucleotide sequence of pACYC184. Nucleic Acids Res. 1988 Jan 11;16(1):355–355. doi: 10.1093/nar/16.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbaken M. G., Culbertson M. R. Mutations in elongation factor EF-1 alpha affect the frequency of frameshifting and amino acid misincorporation in Saccharomyces cerevisiae. Genetics. 1988 Dec;120(4):923–934. doi: 10.1093/genetics/120.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, Edition VI. Microbiol Rev. 1983 Sep;47(3):410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapio S., Isaksson L. A. Antagonistic effects of mutant elongation factor Tu and ribosomal protein S12 on control of translational accuracy, suppression and cellular growth. Biochimie. 1988 Feb;70(2):273–281. doi: 10.1016/0300-9084(88)90071-5. [DOI] [PubMed] [Google Scholar]
- Tapio S., Kurland C. G. Mutant EF-Tu increases missense error in vitro. Mol Gen Genet. 1986 Oct;205(1):186–188. doi: 10.1007/BF02428051. [DOI] [PubMed] [Google Scholar]
- Tate W. P., Kastner B., Edgar C. D., McCaughan K. K., Timms K. M., Trotman C. N., Stoffler-Meilicke M., Stoffler G., Nag B., Traut R. R. The ribosomal domain of the bacterial release factors. The carboxyl-terminal domain of the dimer of Escherichia coli ribosomal protein L7/L12 located in the body of the ribosome is important for release factor interaction. Eur J Biochem. 1990 Feb 14;187(3):543–548. doi: 10.1111/j.1432-1033.1990.tb15335.x. [DOI] [PubMed] [Google Scholar]
- Van de Klundert J. A., Van der Meide P. H., Van de Putte P., Bosch L. Mutants of Escherichia coli altered in both genes coding for the elongation factor Tu. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4470–4473. doi: 10.1073/pnas.75.9.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijgenboom E., Bosch L. Translational frameshifts induced by mutant species of the polypeptide chain elongation factor Tu of Escherichia coli. J Biol Chem. 1989 Aug 5;264(22):13012–13017. [PubMed] [Google Scholar]
- Vijgenboom E., Vink T., Kraal B., Bosch L. Mutants of the elongation factor EF-Tu, a new class of nonsense suppressors. EMBO J. 1985 Apr;4(4):1049–1052. doi: 10.1002/j.1460-2075.1985.tb03737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. B., Dunn D. M., Atkins J. F., Gesteland R. F. Slippery runs, shifty stops, backward steps, and forward hops: -2, -1, +1, +2, +5, and +6 ribosomal frameshifting. Cold Spring Harb Symp Quant Biol. 1987;52:687–693. doi: 10.1101/sqb.1987.052.01.078. [DOI] [PubMed] [Google Scholar]
- Weiss R. B., Murphy J. P., Gallant J. A. Genetic screen for cloned release factor genes. J Bacteriol. 1984 Apr;158(1):362–364. doi: 10.1128/jb.158.1.362-364.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., vanCleemput M. Nucleotide sequence of trpE of Salmonella typhimurium and its homology with the corresponding sequence of Escherichia coli. J Mol Biol. 1982 Mar 5;155(3):235–246. doi: 10.1016/0022-2836(82)90003-1. [DOI] [PubMed] [Google Scholar]