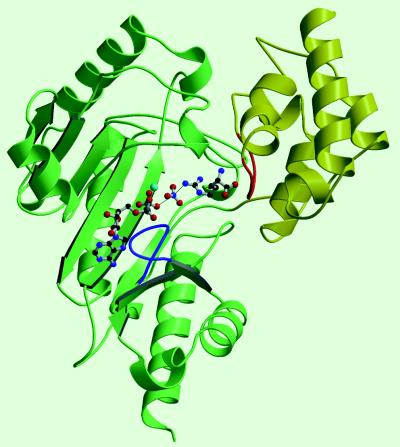
Figure 1.
Structure of AK. The N-terminal domain is shown in yellow and the C-terminal domain in green. The TSA complex ligands, shown in ball-and-stick, lie left to right in the order ADP, Mg2+ (light blue), nitrate, and arginine with arginine bridging between the large domain and a region of the small domain (highlighted in red) that is likely involved with substrate specificity and movement of the small domain. Two loops (63–68, red; and 309–318, blue) that were disordered in the MibCK structure are found in the AK TSA complex in substantially different conformation.