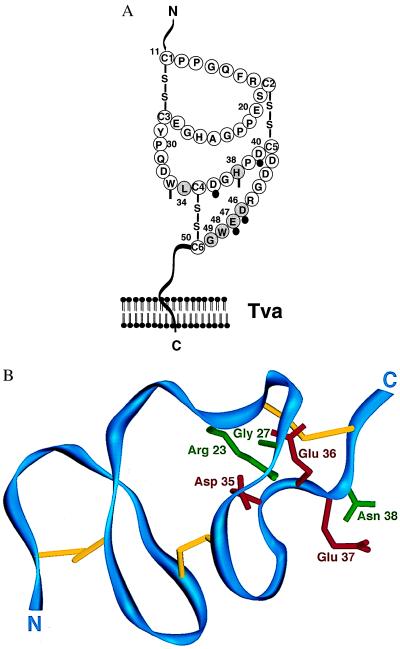
Figure 4.
Amino acids of the Tva LDL-A module critical for viral receptor function. (A) A cartoon of the LDL-A module from Tva. The six residues critical for viral receptor function of Tva are shaded and numbered. Residues of the LDL-A module of Tva corresponding to the residues involved in calcium ion coordination in the hLDL-A5 are indicated. Solid circles denote residues with side chains participating in Ca2+ coordination, and vertical bars indicate the residues with backbone carbonyl oxygens contributing to Ca2+ coordination (18). (B) Mapping residues critical for Tva viral receptor function on the structure of LDL-A5. The backbone of LDL-A5 is shown in blue, and the three disulfide bonds are shown in yellow from an angle similar to that in figure 2 of Fass et al. (18). This structure was generated by the program setor using the coordinates of hLDL-A5 deposited in the Protein Data Bank at Brookhaven National Laboratory. The six residues of hLDL-A5 corresponding to those in the Tva LDL-A module (see A) are labeled: green, the side chains of the corresponding residues of Tva identified by this study, Arg-23(Leu-34), Gly-27(His-38), and Asn-38(Gly-49); red, the side chains of the corresponding residues of Tva identified by previous studies (8, 9), Asp-35(Asp-46), Glu-36(Glu-47), and Glu-37(Trp-48). Shown in parentheses are the corresponding residues in Tva.