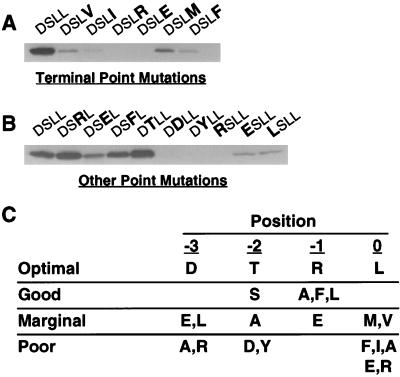
Figure 1.
NHERF preferentially binds to the motif D-S/T-x-L at the end of target proteins. (A) Binding of the first PDZ domain of NHERF to the β2 receptor tail is inhibited by point mutations of the final residue of the tail. GST fusion proteins of the β2 receptor tail with mutations at the terminal position were expressed and purified. Equal amounts (≈25 μg) were loaded on SDS/PAGE gels, blotted, and overlaid with NHERF PDZ1 fusion protein (50 nM). The mutations of the β2 receptor tail (D-S-L-L) are indicated in bold at the top of the panel. (B) Point mutations at the −1, −2, and −3 positions of the β2 receptor tail differentially alter NHERF PDZ1 binding. Equal amounts of various β2 receptor tail point mutants were loaded on an SDS/PAGE gel, blotted, and probed for NHERF PDZ1 fusion protein binding. (C) Summary of NHERF PDZ1-binding preferences at the final four amino acid positions of target proteins based on single amino acid substitution studies of the β2 receptor tail. The optimal amino acid for each position is shown in the first line of the table. “Good” means that the given change causes less than a twofold loss in NHERF PDZ1 binding relative to the optimal amino acid at that position, whereas “marginal” means that the given change causes more than a twofold but less than a 10-fold decrease in binding. “Poor” means that the given change leads to a >10-fold decrease in NHERF PDZ1 binding. The results are representative of two to four independent determinations for binding to each mutant tail.