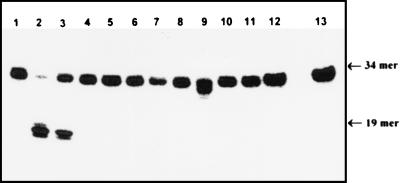
Figure 4.
Action of various E. coli and human DNA repair proteins on the 34-mer duplex ɛC/G oligonucleotide. The 5′-32P-labeled ɛC-34/G was incubated with an excess of the various pure repair protein at 37°C for 30 min (unless otherwise stated). Except for the control ɛC-34/G oligonucleotide and this oligonucleotide treated with Nth, Nfo, or Xth protein, the reactions were made in the presence of Fpg protein (50 ng) to reveal any abasic site generated by DNA glycosylases devoid of β-lyase activity. Lane 1, control ɛC-34/G oligonucleotide. Lane 2, as lane 1, but treated by E. coli dsUDG protein (5 ng). Lane 3, hTDG (150 ng, 30°C). Lane 4, AlkA (400 ng). Lane 5, ANPG40 (1.3 μg). Lane 6, Fpg protein (1 μg). Lane, 7, Nth protein (100 ng). Lane 8, Nfo (1.2 μg). Lane 9, Xth (4 nM, 10 min, 23°C). Lane 10, UNG (85 ng). Lane 11, APDG60 protein (1 μg). Lane 12, Tag I (350 ng). Lane 13, control as 1. The products of the reaction were analyzed as described in Fig. 1. For details see Materials and Methods.