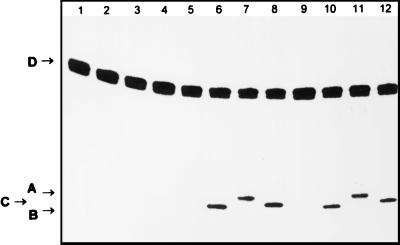
Figure 5.
Mechanism of action of the dsUDG and hTDG proteins on ɛC/G oligonucleotide. The ɛC-34/G duplex oligonucleotide was incubated with dsUDG protein or hTDG protein, and subsequently treated or not with proteins nicking at AP sites to reveal abasic sites generated by dsUDG or hTDG proteins. The 5′-32P-labeled 34-mer ɛC-34/G duplex oligonucleotide is: lane 1, incubated at 37°C for 30 min; lanes 2, 6, and 10, incubated with 100 ng of Fpg protein at 37°C for 10 min; lanes 3, 7, and 11, incubated with 100 ng of Nth protein at 37°C for 10 min; lanes 4, 8, and 12, incubated with 100 ng of Nfo protein at 37°C for 10 min; lanes 5–8, incubated with 2 ng of dsUDG protein at 37°C for 10 min; lanes 9–12, incubated with 50 ng of hTDG protein at 30°C for 30 min. The products of the reaction were analyzed as described in Fig. 1. Arrow A indicates the 19-mer oligonucleotide containing an α,β-unsaturated aldehyde at the 3′-end. Arrow B indicates the 19-mer oligonucleotide containing a phosphate at the 3′-end. Arrow C indicates the 19-mer oligonucleotide containing 3′-OH termini. Arrow D indicates the 34-mer ɛC-34 oligonucleotide. For details see Materials and Methods.