Abstract
Background
Evidence suggests that the transition from experimental to regular smoking is facilitated by the influence of tobacco on affective and attentional mechanisms. The objective of this study was to examine affective and attentional responses in young adult smokers using fear-potentiated startle and prepulse inhibition.
Methods
Participants were 56 college non smokers, non-abstinent smokers, and overnight-abstinent smokers. The fear-potentiated startle test examined phasic responses to imminent threat cues and more sustained responses to unpredictable aversive events. Prepulse inhibition investigated responses to attended and ignored prepulse stimuli.
Results
Abstinent and non-abstinent smokers showed increased sustained potentiation of startle to contextual cues, compared to controls. Abstinent smokers showed increased fear-potentiated startle to threat cues, compared to non-smokers. PPI did not discriminate between abstinent or non-abstinent smokers and controls.
Conclusion
These findings suggest that negative affectivity or anxiety is associated with smoking, particularly during withdrawal. Potentiated startle may provide a valuable tool in understanding the biologic mechanisms underlying nicotine withdrawal and inform cessation and prevention efforts.
Keywords: Smoking, nicotine, fear-potentiated startle, prepulse inhibition, affective, anxiety
Introduction
The association between tobacco and affective regulation and disorders is well-established (Breslau 1995; Pomerleau and Pomerleau 1987). Affective and cognitive processes facilitate the transition from tobacco use to dependence (DiFranza et al. 2004), and are also associated with acute withdrawal from nicotine (Hughes et al 1994). The results of most studies reveal that smokers report alleviation of negative affect by smoking, (Brandon and Baker 1991) and increased anxiety during withdrawal. Nicotine also has a stimulant effect on cognitive processes, improving vigilance, enhancing selective attention, and promoting response inhibition to irrelevant stimuli (Jones et al. 1992).
Whereas some theories of the association between smoking and anxiety states are based on the anxiety-reduction model of smoking, others (Parrott 1999) propose that the apparent reduction in anxiety after a cigarette is due to mood normalization after short-term withdrawal symptoms. These two explanations generate different experimental predictions: (1) the anxiety reduction hypothesis predicts that smokers have greater basal reactivity to stressors, perhaps more so to distal/unpredictable stressors than to proximal stressors (see below) than non-smokers irrespective of their withdrawal status; and (2) the normalization of withdrawal explanation predicts augmented reactivity to stressors during abstinence.
Measurement of the psychological effects of smoking has been limited by the reliance on self-reports of subjective experience which has yielded inconsistent findings (West and Hajek 1997). Therefore, objective measures of the response to nicotine that test emotional and cognitive changes may elucidate the processes underlying nicotine dependence. The two components of the startle reflex that may be used to test affective and cognitive responses to nicotine are fear-potentiated startle and prepulse inhibition.
Fear-potentiated startle
An objective measure of affective reactivity is the fear-potentiated startle reflex, which tests the increase in startle magnitude during states of negative affectivity or anxiety (Davis 1986; Grillon and Baas 2003). In both humans and animals, two distinct types of aversive states can be identified in anticipation of aversive stimuli (e.g., electric shocks): a phasic fear response to a proximal threat and a more sustained anxiety state induced by more distal or unpredictable stressors (see (Davis 1998; Grillon and Baas 2003), which are mediated by distinct neural structures (the amygdala and the bed nucleus of the stria terminalis, respectively) (Davis 1998). These two aversive states are relevant to smoking addiction because it has been proposed that nicotine have a differential anxiolytic effect on distal and proximal threats (Gilbert 1995; Kassel et al. 2003). Smoking has been found to reduce anxiety to distal or uncertain and ambiguous stressors (Gilbert et al. 1989), but not to proximal stressors (Fleming and Lombardo 1987).
Prepulse inhibition
Smoking is also associated with improved cognitive processing. Prepulse inhibition (PPI), the reliable reduction in the magnitude of the startle reflex when a startling probe is preceded immediately by a weak stimulus (prepulse) (Geyer et al. 2001), is an operational measure of sensory gating, i.e., the ability to screen out sensory stimuli, and a measure of changes in central neuronal functioning (Geyer et al. 2001). Previous findings on the association between PPI and smoking are contradictory (Faraday et al. 1999; Hutchison et al. 2000); studies of both rodents and humans have found that nicotine can increase (Acri et al. 1994; Duncan et al. 2001; Kumari et al. 1996) or decrease PPI (Faraday et al. 1999; Hutchison et al. 2000). Because attention can modulate PPI (Filion et al. 1993), it is possible that discrepancies between studies are due to differences in the level of attention to the prepulse across studies.
The present study
The present study was designed to identify potential biological markers for regular tobacco use in young adults. By early adulthood, most youth have passed through two periods of heightened risks for tobacco use – adolescence and the transition to college or the work environment -- and sufficient time has elapsed to establish regular smoking patterns and nicotine dependence (Breslau et al. 2001). The specific aims of the present study were: (1) to compare measures of anxiety using fear-potentiated startle; and (2) to examine differences in information processing and attention using pre-pulse inhibition (PPI) in abstinent and non-abstinent smokers compared to non smokers.
Consistent with the anxiety reduction model, we predicted that increased vulnerability to stress in smokers would be reflected in an increase in fear-potentiated startle, whereas a causal model of smoking and anxiety would predict that smokers would show elevated fear-potentiated startle only during abstinence. Based on the hypothesis that nicotine facilitates information processing and attention, we predicted that the abstinent smokers would show reduced PPI compared to the two other groups. In addition, because PPI is affected by attention to the prepulse, we built an attend/ignore-the-prepulse instruction into the design of the present study (Hutchison et al. 2003)
Materials and methods
Subjects
The sample consisted of 56 current smokers (21 males and 17 females) and non-smokers (7 males and 11 females), ages 18-26 years, participated in the study. Seven subjects with no startle on most trials were excluded from the analyses. Subjects were screened for vision, hearing, psychiatric, and neurological conditions as well as current medication use.
Inclusion criteria for the non-smoker group were: 1) exposure to at least one cigarette during their lifetime, 2) no smoking in the last 6 months, 3) smoked less than 100 cigarettes in their lifetime, and 4) never daily smokers. The inclusion criteria for the smoker group were: 1) current daily smoking, 2) smoking at least 5 cigarettes per day for the last 6 months, and 3) symptoms of DSM-IV nicotine dependence. The smokers were randomly divided into an abstinent and non-abstinent smoker groups. The abstinent smokers were deprived of smoking overnight and did not smoke prior to the test. The non-abstinent smokers were allowed to smoke freely. They were also asked to smoke a cigarette ½ hour prior to the test. The subjects participated in the study after signing informed consent approved by our Human Investigation Committee.
Procedure
Three tests were used: startle habituation, to reduce excessive initial startle reactivity, which was followed by a threat experiment, and finally a PPI experiment.
Stimuli and Physiological Responses
Stimulation and recording were controlled by a commercial system (Contact Precision Instruments, London, Great Britain). The acoustic startle stimulus was a 40-ms duration white noise presented binaurally through headphones. The intensity of the startle stimuli was 103 dB in the threat experiment and 115 dB in the PPI experiment. The eyeblink reflex was recorded with electrodes placed under the left eye. The electromyographic signal was amplified with bandwidth set to (30-500 Hz) and digitized at 1000 Hz. In the threat experiment, the airblast was an intense (80 psi) jet of air delivered by plastic tubing at the level of the larynx as described in (Grillon and Ameli 1998).
Startle habituation
Subjects were presented with eight startle stimuli delivered every 18-25 sec.
Threat experiment
Details of the procedures are provided elsewhere (Grillon et al. 2004). Participants received explicit instructions regarding the conditions under which the airblasts would be delivered. As shown in Fig. 1, the experiment consisted of three different conditions: no airblast (N), and predictable (P) and unpredictable (U) airblast, each lasting approximately 90 sec. In the N condition, no airblasts were delivered. In the P condition, airblasts were administered predictably only in the presence of a threat cue. In the U condition, the airblasts were unpredictable. In each 90-sec condition, an 8-sec cue was presented twice. The cues were geometric colored shapes in the different conditions (e.g., blue square for N, red circle for P). The cue signaled the possibility of receiving an airblast in the P condition, but it had no signal value in the N and U conditions. Instructions were displayed on a computer monitor to inform participants of the current condition by displaying the following information throughout the condition: “no airblast” (N), “airblast only during shape” (P), or “airblast at any time” (U). In each N, P, and U condition, four acoustic startle stimuli were delivered. Two startle stimuli were delivered during inter-trial intervals (ITI; i.e., between cues) to assess contextual anxiety and one during each of the two cues, 5-7 sec following the cue onset to assess fear to the cue. In each P condition, one airblast was administered during one of the two cues (at cue offset). In each U condition, one airblast was delivered in the absence of cue. The threat experiment consisted of two recording blocks with a 5-10 min rest between blocks. Each block consisted of three N, two P, and two U conditions in one of the following two orders: P N U N U N P or U N P N P N U. Each participant was presented with the two orders, with half the participants starting with the P condition.
Fig. 1.
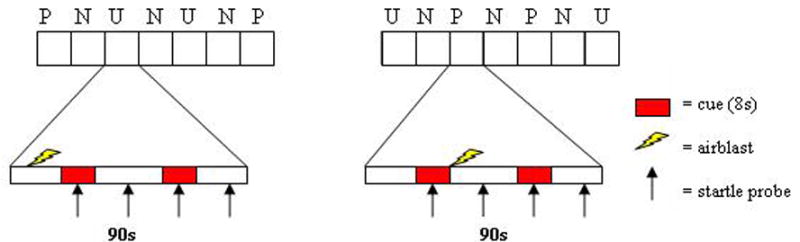
Schematic diagram of the fear-potentiated startle experiment. Airblast were administered in 90-sec duration predictable (P) and the unpredictable (U) context but not during the 90-sec duration no-airblast (N) contexts. There were two 8-sec duration cues in each context (cues in N context are not shown in the graph). Airblasts were administered during a cue in the predictable condition and in the absence of cues in the unpredictable condition. A single airblast was delivered in each predictable and unpredictable context. Startle stimuli were delivered in the presence of each cue. Two startle stimuli were also delivered in the absence of cues (during intertrial intervals or ITI) in each 90-sec context. Potentiation of startle during a cue relative to during the absence of a cue was taken as the expression of cued fear. Potentiation of startle in the absence of the cue in the P and U condition relative to the N condition was taken as the expression of contextual anxiety. There were two sequences of stimuli, 1) PNUNUNP and 2) UNPNPNU. Subjects were presented with each of these sequences either with the sequence starting with P first or the sequence starting with U first.
Subjective mood rating
Subjects were asked to rate how anxious, calm, energetic, and drowsy they felt during each of the conditions after each threat block using an analog scale ranging from 1 (not at all) to 5 (extremely). We did not attempt to separate responses during the cues and in the absence of cue.
PPI experiment
The experiment was a 2 (attend, ignore) × 2 (short tone, long tone) × 2 (control startle, prepulse) design as depicted in Fig. 2. There were two types of 1000 Hz tones (75 dB), short lasting 5 sec, and long lasting 6 sec. Startle stimuli (115 dB) were presented at the onset of the 1000-Hz tone on control startle trials or 120 ms after the onset of the 1000-Hz tone on prepulse trial. On each trial, participants were asked to attend to the stimuli and to report verbally whether they heard a short- or long-duration 1000 Hz tone, or to ignore the tones. Approximately 7 sec before each startle/tone trials, the words “attend” or “ignore” appeared on a monitor placed in front of the subjects to instruct subjects to count or not count the number of short/long tones.
Fig. 2.
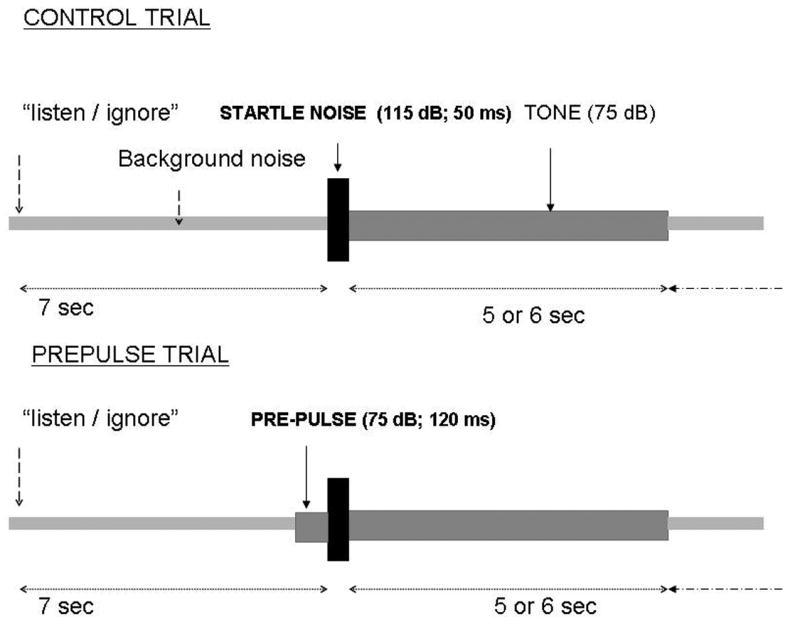
Schematic diagram of the PPI experiment. On control trials, acoustic startle stimuli were delivered at the onset of 5- or 6-sec duration tones. On prepulse trials, acoustic startle stimuli were delivered 120 sec after the onset of the tones. Seven sec before a tone onset, the words “listen” or “ignore” were displayed on a computer monitor. On “attend” trials, subjects had to listen to the tones and report the number of short- and long-duration tones.
The PPI experiment started with six startle stimuli (without the 1000 Hz tone) to reduce initial startle reactivity. This was followed by two PPI blocks trials. Each block consisted of six control startle trials (4 associated with a 6-sec tone and 2 associated with a 5-sec tone) and six prepulse trials (4 associated with a 6-sec tone and 2 associated with a 5-sec tone), such that there were three attend/control, three attend/prepulse, three ignore/control, and three ignore/prepulse trials. Each subject was presented with one of four sequences of trials. In each sequence, the different trials were organized in a pseudo-random manner. There were no more than two successive attend or ignore conditions and there were no more than two successive presentations of trials of the same category (control or PPI trials). The subjects of each group were distributed approximately evenly among the four sequences.
Clinical, smoking, and affect measures
DSM-IV symptoms of nicotine dependence were assessed via a structured interview with the Tobacco Use section of the World Mental Health-Composite International Diagnostic Interview (Kessler and Üstün 2004). The severity of nicotine dependence was assessed with the Fagerstrom Tolerance Questionnaire (Fagerstrom and Schneider 1989). Smoking status was measured by self-report questionnaires and expired carbon monoxide using a Smokerlyzer (Bedfont Scientific Ltd, Rochester, England). Carbon monoxide (CO) was measured just after arrival in the laboratory (before the non abstinent smokers smoke). Just prior to placing the electrodes, subjects were asked to rate their urge to smoke (smokers only) with a visual analog scales (100 mm, VAS); and their subjective mood using the Positive and Negative Affect Schedule (PANAS), a self-administered scale that measures two broad distinct dimensions of ‘affect’, one ‘positive’ and the other ‘negative’ (Watson et al. 1988).
Data Analysis
Response magnitude of the blink reflex was defined as the maximum of the response in a 20-100-ms time frame following stimulus onset relative to baseline (average baseline EMG level for the 50 ms immediately preceding stimulus onset). For habituation, peak magnitudes were averaged over two successive trials. The habituation data were analyzed with a Group (non smokers, smokers, abstinent smokers) × Sex (males, females) × Block (4 blocks) ANOVA. The startle magnitude data during the threat experiment were averaged within conditions across blocks. Given that the experiment was designed to examine phasic cued fear and sustained anxiety, two types of analyses were conducted. Cued fear was operationally defined as fear-potentiated startle during the cues. Startle potentiation during cues was obtained by calculating difference scores between startle magnitude during the cue and startle magnitude in the absence of the cue (during ITI). Sustained anxiety was operationally defined as the potentiation of startle during ITI in the two threat conditions, relative to the no-airblast condition. The cued fear and sustained anxiety scores were then analyzed using separate three-way ANOVA with Group (non smokers, smokers, abstinent smokers) and Sex (males, females) as between-group factors and Condition (neutral, predictable, unpredictable) as repeated factors. To evaluate the source of the significant main effect of Condition, a linear trend in startle magnitude over N, P, and U was tested. The startle data during the PPI experiment were analyzed with Group (non smokers, smokers, abstinent smokers) × Sex (males, females) × Trial Type (control, prepulse) × Condition (attend, ignore) ANOVA. The data were averaged over trials associated with different duration 1000 Hz tones, since the duration of the tone could not affect startle to the startle noise. Performance during the PPI experiment was expressed as the total of correct responses (i.e., correct identification of short and long prepulse). The data were analyzed with a Group (non smokers, smokers, abstinent smokers) × Sex (males, females) ANOVA. Alpha was set at .05 for all statistical tests. Analyses of covariance (ANCOVA) were also used to examine the effect of variables on which there were group differences Greenhouse-Geisser corrections (GG-ε) were used for main effects and interactions involving factors with more than two levels.
Results
Group Characteristics
Table 1 presents demographic, smoking history, and positive/negative affect information for each group. There were 41 males and 32 females and the average age of the sample of 21 did not differ across groups. The sex and age distribution did not differ among the study subgroups. The data were analyzed with one-way ANOVAs comparing either the three groups or the two smoking groups. A significant Group main effect was followed up by pairwise comparisons when appropriate. Carbon monoxide levels differed across the three groups (F(2,52)=33.44, p<.0001), supporting the assumption that nonsmokers had not smoked recently and that abstinent smokers abstained from smoking in the past 12 hours. There were no significant group differences in positive or negative affect (F(2,52)=.98, p=.38 and F(2,52)=.62, p=.54, respectively).
Table 1. Means (standard deviations) of demographic, smoking, affect variables by smoking group.
| Demographic and Smoking Measures | Nonsmokers | Abstinent Smokers | Non Abstinent Smokers | P value | |
|---|---|---|---|---|---|
| n=18 | n=16 | n=22 | Group | Abstinence | |
| Age (yrs) | 21.92 (2.89) | 21.00 (1.70) | 20.59 (2.02) | ns | ns |
| Sex (% male) | 60.1% | 50% | 55% | ns | ns |
| # Cigarettes per day (past 30 days) | 0.00 | 12.19 (6.01) | 13.84 (5.88) | NA | ns |
| CO level (ppm) at arrival | 1.53 (0.87) | 3.87 (1.86) | 12.14 (6.53) | <.001 | ns |
| Fagerstrom Tolerance Questionnaire | - | 3.44 (2.31) | 3.00 (2.20) | NA | ns |
| Age of onset daily smoking | -- | 18.40 (1.76) | 17.14 (1.61) | NA | .03 |
| Urge to smoke | 1.07 (0.10) | 4.58 (1.51) | 4.24 (1.11) | ns | ns |
| PANAS | 27.00 (10.52) | 26.81 (8.34) | 23.54 (7.44) | ns | ns |
| Positive affect (state) | |||||
| PANAS | 12.18 (3.13) | 13.19 (2.93) | 12.36 (2.40) | ns | ns |
| Negative affect (state) | |||||
See text for detailed statistics
All smokers reported DSM-IV symptoms of nicotine dependence, although Fagerstrom Tolerance Questionnaire scores were somewhat low (mean = 3.2). The smokers smoked an average of 13 cigarettes per day (past 30 days). The two smoker groups did not differ on the average number of cigarettes smoked per day (F(1,36)=.72, p=.40), on Fagerstrom Tolerance Questionnaire scores (F(1,36)=.35, p=.56), or on the urge to smoke (F(1,36)=0.651, p=0.43). The age of onset of daily smoking was slightly but significantly higher in the abstinent smokers compared to the non-abstinent smokers (F(1,35)=5.08, p<.03).
Startle habituation
Overall startle magnitude during habituation did not differ among non smokers, smokers, and abstinent smokers (F(2,50)=.79; 18.4 (sem = 3.7), 17,1 (sem = 3.3), 23.2 (sem = 3.8), respectively). Startle habituated across blocks (F(3,150)=2.7, p<.05, epsilon = .92), but the rate of habituation did not differ significantly among groups.
Threat experiment: Fear-potentiated startle
Cued fear
Fig. 3 shows the fear-potentiated startle data to the cues, expressed as the difference scores between startle during cues minus startle during ITI. The Condition main effect was significant (F(2,100)=19.3, p<.0009), reflecting, as expected, greater startle potentiation during the threat cue in the predictable condition, compared to the two no airblast and unpredictable conditions. There was also a significant Group main effect (F(2,50)=3.2, p<.05). Pairwise comparisons showed significantly greater fear-potentiated startle during the cue (irrespective of conditions) in the abstinent smokers compared to the non-smokers (p=.05). The non-abstinent smokers did not differ significantly from the non smokers and the abstinent smokers (p>.1). Finally, females had greater fear-potentiated startle during the cues compared to males (F(1,50)=5.5, p=.02). The Group × Condition interaction was not significant (F(4,100)=1.1),
Fig. 3.
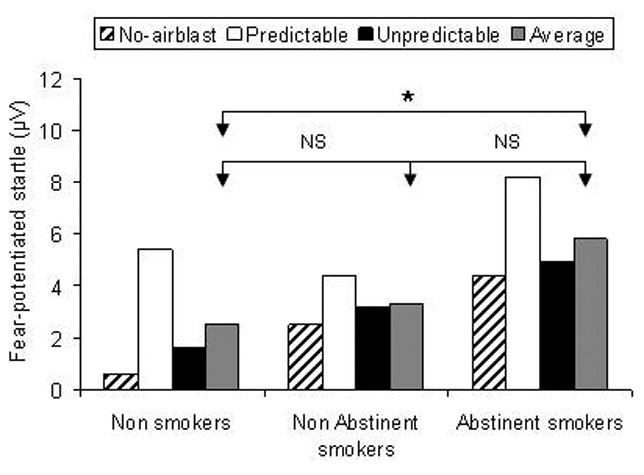
Fear-potentiated startle to the cue in each condition. The data show the difference scores between startle during the cues minus startle during ITI (in the absence of cues) in the no-airblast, predictable, and unpredictable condition. The average score across these three conditions is also shown. * for significant effect (p<.05). NS for non significant.
Contextual anxiety
The startle data during ITI are shown in Table 2. Overall startle magnitude during ITI did not differ significantly among groups (F(2,50)=.37). Startle magnitude during ITI increased from the neutral, to the predictable, and to the unpredictable conditions (Condition: F(2,100)=11.9, p=.0009, GG-ε =.89; linear trend: F(1,50)=26.0, p=.0009). However, this effect was mainly due to the smokers, as evidenced in a Group × Condition interaction (F(2,100) = 2.5, p=.05, GG-ε =.89) and a near Group × Condition linear trend (F(2,50)=10.2, p=.06). Because we were interested in the potentiation of startle in the two threat conditions, we calculated “contextual startle potentiation scores” for the predictable (ITI startle magnitude during predictable condition minus ITI startle magnitude during the neutral condition) and the unpredictable condition (ITI startle magnitude during unpredictable condition minus ITI startle magnitude during the neutral condition) (Fig. 4). These scores were compared among groups using t-tests. Startle potentiation during ITI in the predictable condition was larger in the non-abstinent smokers compared to the non smokers (t(38)=2.2, p<.04). There was no significant difference between the non smokers and the abstinent smokers in the predictable condition. Startle potentiation during ITI in the unpredictable condition was larger in the abstinent smokers (t(32)=2.1, p<.04) and tended to be larger in the non-abstinent smokers (t(38)=1.9, p<.06) compared to the non smokers. There was no significant difference between the two smoker groups.
Table 2. Means (and SEM) startle magnitude (μV) in the fear-potentiated startle experiment.
| CONDITION | Stimulus type | Non smokers | Non abstinent smokers | Abstinent smokers |
|---|---|---|---|---|
| Habituation | 14.6 (2.7) | 15.6 (2.9) | 19.7 (3.7) | |
| No airblast | ITI | 12.3 (2.6) | 11.9 (2.5) | 15.4 (3.1) |
| Cue | 12.9 (2.3) | 14.4 (2.5) | 19.8 (3.4) | |
| Predictable airblasts | ITI | 13.6 (2.81) | 14.9 (2.6) | 16.4 (2.8) |
| Cue | 19.0 (3.5) | 19.7 (2.6) | 24.6 (3.2) | |
| Unpredictable airblasts | ITI | 13.0 (2.4) | 14.1 (2.6) | 18.1 (3.0) |
| Cue | 14.7 (2.3) | 17.3 (2.7) | 22.7 (3.3) |
Fig. 4.
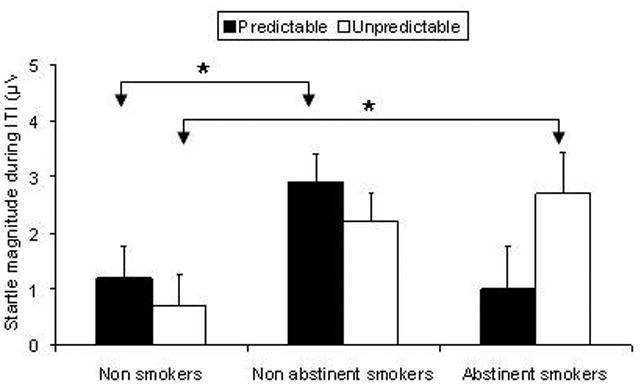
Potentiation of startle during ITI in the predictable and unpredictable conditions. The bar graphs show the difference scores in ITI startle magnitudes during the predictable and unpredictable conditions minus startle magnitude during the no-airblast condition. * for significant effect (p<.05). Note that there was a trend (p<.06) for greater startle potentiation in the unpredictable condition between the non-abstinent smokers compared to the non-smokers.
Threat experiment: Subjective reports
The retrospective subjective mood ratings during each condition are shown in Table 3. Each mood rating was analyzed using separate Condition × Group × Sex ANOVAs. Subjects felt progressively more anxious (linear trend: F(1,50)=83.5, p<.0009), less calm (linear trend: F(1,50)=52.1, p<.0009), more energetic (linear trend: F(1,50)=15.5, p<.0009), and less drowsy linear trend: F(1,50)=41.6, p<.0009) from the neutral, to the predictable, to the unpredictable condition. These ratings did not differ significantly among groups as demonstrated by the lack of significant Condition × Group or Condition × Group × Sex interactions. The startle and subjective measure remained the same when age of onset of smoking was used as a covariate.
Table 3. Means (and standard deviations) of retrospective mood and aversiveness ratings by smoking group.
| MOOD | Conditions | Nonsmokers | Abstinent Smokers | Non Abstinent Smokers |
|---|---|---|---|---|
| n=17 | n=16 | n=22 | ||
| Anxiety | Neutral | 1.5 (.5) | 1.9 (1.1) | 1.4 (0.6) |
| Predictable | 2.4 (1.0) | 2.3 (0.9) | 2.2 (0.8) | |
| Unpredictable | 2.8 (1.1) | 2.9 (1.2) | 2.6 (0.9) | |
| Calm | Neutral | 3.5 (1.0) | 3.2 (1.2) | 3.7 (0.8) |
| Predictable | 3.0 (1.1) | 3.0 (1.1) | 3.0 (1.1) | |
| Unpredictable | 2.6 (1.1) | 2.8 (1.1) | 2.6 (0.7) | |
|
| ||||
| Energetic | Neutral | 1.6(1.0) | 1.7 (0.8) | 1.7(0.7) |
| Predictable | 1.8 (1.1) | 2.0 (0.9) | 2.0 (0.7) | |
| Unpredictable | 2.1 (1.2) | 2.2 (1.1) | 2.6 (0.7) | |
|
| ||||
| Drowsy | Neutral | 3.1 (1.1) | 3.4 (1.3) | 3.3 (1.0) |
| Predictable | 2.5 (0.9) | 3.0 (1.0) | 2.9 (0.9) | |
| Unpredictable | 2.3 (0.9) | 3.0 (1.1) | 2.6 (0.9) | |
Prepulse inhibition experiment
Results are shown in Table 4. Startle was reliably inhibited by the prepulse stimulus (F(1,47)=17.6, p<.0009). There was also a small but significant increase in prepulse inhibition in the attend compared to the ignore condition (F(1,47)=7.0, p<.01). PPI scores did not differ significantly among groups as reflected by the lack of Group × Stimulus Type (F(2,47)=1.1) or Group × Stimulus Type × Condition (F(2,47)=.2) interactions. Consistent with previous results, PPI was greater in males compared to females (Sex × Stimulus: (F(1,47)=4.3, p<.04) (results not shown). Results were similar when the PPI data were expressed as percent scores.
Table 4. Means (SEM) startle magnitude (μV) in the PPI experiment.
| INSTRUCTION | Stimulus type | Non smokers | Non abstinent smokers | Abstinent smokers |
|---|---|---|---|---|
| Attend | Control startle | 15.3 (12.1) | 16.5 (13.2) | 23.8 (19.1) |
| Prepulse | 10.3 (9.0) | 10.0 (12.6) | 11.5 (12.4) | |
| PPI* | 5.0 (10.3) | 6.4 (5.1) | 12.2 (15.9) | |
| Ignore | Control startle | 14.0 (11.7) | 15.0 (11.9) | 23.1 (17.0) |
| Prepulse | 10.5 (8.9) | 11.3 (13.6) | 12.5 (13.0) | |
| PPI* | 3.4 (10.6) | 3.6 (6.0) | 10.5 (15.1) |
PPI was calculated as the difference score between control startle trials and prepulse trials
Regarding the performance data, the non-smokers showed a reduced percent of correct identification of long- and short-duration target tones compared to the non-abstinent smokers (mean = 67.6%, SD = 15.2% and mean 79.9%, SD = 11.9%, respectively). This result was confirmed by a significant group main effect (F(2,57)=3.4, p<.04). Pairwise comparisons showed a significant reduced performance in the abstinent smokers compared to the non-abstinent smokers (F (2,57)=11.8, p<.04). The non smokers (mean = 75.6%, SD = 14.9%) did not differ significantly from any of the two smoker groups. The PPI results remained the same when age of onset of smoking was used as a covariate.
Discussion
The major findings of the present study are that: 1) smokers (both abstinent and non-abstinent) had greater sustained potentiated startle than non-smokers during the unpredictable condition, and 2) acutely abstinent smokers had greater cue-potentiated startle than non-smokers. In contrast, pre-pulse inhibition of startle did not differentiate between smokers and non-smokers. These findings suggest that the potentiated startle paradigm may have utility as an objective measure of the association between smoking and affective traits and states.
Sustained anxiety in smokers vs. nonsmokers
The increase in contextual anxiety among smokers compared to non-smokers suggests that smokers have greater anxiety reactivity than non smokers. The responses of smokers to the potentiated startle paradigm are remarkably similar to those of individuals with anxiety disorders and children at risk for the development of anxiety disorders (Grillon et al. 1997; Grillon et al. 1998; Lissek et al. 2005). The lower threshold for anxiety among smokers suggests that smokers may overreact to stimuli that are only mildly challenging to non smokers (Grillon et al. 2004). This is consistent with the contention that nicotine alleviates the response to distal/uncertain threat, but not response to proximal threat, and the hypothesis that smokers are constitutionally more anxious or neurotic (Gilbert and Gilbert 1995; Goodwin and Hamilton 2002) than non smokers. On the other hand, it is also possible that anxiety is increased due to early withdrawal in abstinent smokers and that trait anxiety is elevated in the non-abstinent smokers (West and Hajek 1997). Future studies should therefore examine not only the potential explanations for the role of trait anxiety as a vulnerability factor for regular smoking, but also the role of smoking on affective reactions.
Fear potentiated startle to cues in abstinent smokers
Potentiated startle to cues was significantly elevated among abstinent smokers compared to non-smokers in response to all cues rather than solely to the predictable cues. This effect may either be attributable to cigarette withdrawal symptoms (Hughes et al. 1994; Parrott and Garnham 1998), which increase sensitivity to stimuli with negative valence (Cinciripini et al. 2006). Alternatively, if nicotine is used as an anxiolytic agent (Shiffman 1982), abstinence may uncover underlying anxiety in smokers during acute withdrawal. The finding that non-abstinent smokers exhibited normal fear-potentiated startle to a threat cue but elevated sustained startle potentiation during ITI in the unpredictable condition, suggests that the feature of the stressor may determine abnormal affective responses in smokers (Gilbert 1995).
Comparison with other fear-potentiated startle studies
Two other studies have examined the effect of nicotine withdrawal on fear-potentiated startle (Geier et al. 2000; Hogle and Curtin 2006). However, the methods of the two studies differed so substantially from those of the present study that comparability with our findings are limited. Whereas the unpleasant pictures did not faciliate startle in either the smokers or controls in the former study, the airblast in the present study facilitated startle in all three experimental groups. Likewise, the Hogle and Curtin (2006) study used a different method by comparing startle potentiation during safe and threat conditions rather than threat versus no cue as in the present study. Because smokers tend to generalize across conditions, Hogle and Curtin may have underestimated the magnitude of fear-potentiated startle to the threat cue.
Subjective responses
The lack of concordance between the subjective reports and the startle findings has been reported in numerous prior studies (e.g., Grillon et al in press). The most likely reason for this discrepancy is the difference in the timing of the two measures; whereas the startle probe is a direct in vivo measure of anxiety, the self report ratings were obtained after the completion of the experiment. Alternatively, startle may be a more sensitive measure than subjective ratings, or the two measures may reflect distinct processes. This latter explanation is consistent with findings that the amygdala can be activated without alteration in conscious mood (Morris et al. 1998).
Pre-pulse inhibition in smokers vs. non-smokers
In the present study, PPI did not distinguish between smokers and non-smokers nor between abstinent and non-abstinent smokers. The only finding that discriminated between groups was a deficit in target identification in the abstinent smokers during the PPI test. This result is consistent with findings of disruption of attention following tobacco withdrawal (Jacobsen et al. 2005). The lack of differences in PPI among the three groups in the present study, suggests that PPI may not be a useful objective measure of smoking vulnerability or progression.
Strengths and Limitations
These findings should be interpreted in the context of the strengths and limitations of this study. The majority of studies in humans assessing the relationships between negative affectivity/anxiety, stress and smoking have relied on verbal reports, which are vulnerable to task demands, intentional distortion, and lack of awareness of subliminal effects. The fear-potentiated startle and PPI in this study provided more objective measures of affect and cognition than earlier studies that were based on self reported interpretation of these states. In addition, due to the translational nature of startle experimentation, fear-potentiated startle and PPI provide tools to bridge the gap between pre-clinical and clinical research, and vice versa because of the well-established neural pathways underlying these measures. Another strength of this study was the use of a population-based sample of young adults who had already had a history of smoking, thereby enhancing the generalizability of the findings.
Study limitations include: the relatively small number of subjects tested; the potential lack of generalizability to younger children or older adults; and the cross-sectional design, which precluded our ability to test the stability of the findings over time. However, some of the findings (e.g., increased sustained anxiety in the non-abstinent smokers) are consistent with hypotheses based on adult samples (Gilbert 1995). Other limitations include the use of only one interval (120 ms) in the PPI study, the lack of counterbalancing of the three experiments in the current study may have led to less sensitivity of the PPI test, the relatively brief withdrawal period, and the lack of control for phase of the menstrual cycle in women in the PPI experiment (Swerdlow et al. 1993).
Implications for research and intervention
The present findings have implications for future research. First, startle could be used to enhance our understanding of the neurobiology underlying the transition from use to dependence as well as withdrawal from nicotine since the structural brain correlates of prepulse inhibition of the acoustic startle response and of sustained startle potentiation have been identified in animal studies and human neuroimaging studies (Swerdlow et al. 2001). Second, if the association between affective reactivity and smoking progression and withdrawal is confirmed in prospective studies, startle could be used an objective test of smoking progression vulnerability or ability to succeed in smoking cessation programs.
Acknowledgments
This study was supported by the Robert Wood Johnson Research Network on the Etiology of Tobacco Dependence, Richard Clayton, Ph.D., Chair, and by the Intramural Research Program of the National Institutes of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acri JB, Morse DE, Popke EJ, Grunberg NE. Nicotine increases sensory gating measured as inhibition of the acoustic startle reflex in rats. Psychopharmacology. 1994;114:369–374. doi: 10.1007/BF02244861. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Baker TB. The Ssmoking Consequences Qestionnaire: The subjective expected utility of smoking in college students. Psychological Assessment. 1991;3:484–491. [Google Scholar]
- Breslau NN. Psychiatric comorbidity of smoking and nicotine dependence. Behavior Genetics. 1995;25:95–101. doi: 10.1007/BF02196920. [DOI] [PubMed] [Google Scholar]
- Breslau NN, Johnson EEO, Hiripi EE, Kessler RR. Nicotine dependence in the United States: prevalence, trends, and smoking persistence. Archives of general psychiatry. 2001;58:810–816. doi: 10.1001/archpsyc.58.9.810. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Robinson JD, Carter BL, Lam C, Wu X, de Moor CA, Baile WF, Wetter DW. The effects of smoking deprivation and nicotine administration on emotional reactivity. Nicotine Tob Res. 2006;8:379–92. doi: 10.1080/14622200600670272. [DOI] [PubMed] [Google Scholar]
- Davis M. Pharmacological and anatomical analysis of fear conditioning using the fear-potientiated startle paradigm. Behavioral Neuroscience. 1986;100:814–824. doi: 10.1037//0735-7044.100.6.814. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biological Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Rigotti NA, Ockene JK, McNeill AD, Coleman M, Wood C. Trait anxiety and nicotine dependence in adolescents: a report from the DANDY study. Addictive Behaviors. 2004;29:911–919. doi: 10.1016/j.addbeh.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Duncan E, Madonick S, Chakravorty S, Parwani A, Szilagyi S, Efferen T, Gonzenbach S, Angrist B, Rotrosen J. Effects of smoking on acoustic startle and prepulse inhibition in humans. Psychopharmacology (Berl) 2001;156:266–72. doi: 10.1007/s002130100719. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO, Schneider NG. Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. Journal of Behavioral Medicine. 1989;12:159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- Faraday MM, O'Donoghue VA, Grunberg NE. Effects of nicotine and stress on startle amplitude and sensory gating depend on rat strain and sex. Pharmacology Biochemistry and Behavior. 1999;62:273–284. doi: 10.1016/s0091-3057(98)00159-2. [DOI] [PubMed] [Google Scholar]
- Filion DL, Dawson ME, Schell AM. Modification of the acoustic startle-reflex eyeblink: A tool for investigating early and late attentional processes. Biological Psychology. 1993;35:185–200. doi: 10.1016/0301-0511(93)90001-o. [DOI] [PubMed] [Google Scholar]
- Fleming SE, Lombardo TW. Effects of cigarette smoking on phobic anxiety. Addict Behav. 1987;12:195–8. doi: 10.1016/0306-4603(87)90027-x. [DOI] [PubMed] [Google Scholar]
- Geier A, Mucha RF, Pauli P. Appetitive nature of drug cues confirmed with physiological measures in a model using pictures of smoking. Psychopharmacology (Berl) 2000;150:283–91. doi: 10.1007/s002130000404. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–54. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Gilbert DG. Smoking: Individual differences, psychopathology, and emotion. Taylor and Francis; 1995. [Google Scholar]
- Gilbert DG, Gilbert BO. Personality, psychopathology, and nicotine response as mediators of the genetics of smoking. Behavior Genetics. 1995;25:133–147. doi: 10.1007/BF02196923. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Robinson JH, Chamberlin CL, Spielberger CD. Effects of smoking/nicotine on anxiety, heart rate, and lateralization of EEG during a stressful movie. Psychophysiology. 1989;26:311–320. doi: 10.1111/j.1469-8986.1989.tb01924.x. [DOI] [PubMed] [Google Scholar]
- Goodwin R, Hamilton SP. Cigarette smoking and panic: the role of neuroticism. The American Journal of Psychiatry. 2002;159:1208–1213. doi: 10.1176/appi.ajp.159.7.1208. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R. Effects of threat and safety signals on startle during anticipation of aversive shocks, sounds, and airblasts. Journal of Psychophysiology. 1998;12:329–337. [Google Scholar]
- Grillon C, Baas JM. A review of the modulation of startle by affective states and its application to psychiatry. Clinical Neurophysiology. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behav Neurosci. 2004;118:916–24. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Grillon C, Dierker L, Merikangas K. Startle modulation in children at risk for anxiety disorders and/or alcoholism. Journal of American Academic Child Adolescent Psychiatry. 1997;36:925–932. doi: 10.1097/00004583-199707000-00014. [DOI] [PubMed] [Google Scholar]
- Grillon C, Dierker L, Merikangas KR. Fear-potentiated startle in adolescents offspring at risk for anxiety disorder. Biological Psychiatry. 1998;44:990–997. doi: 10.1016/s0006-3223(98)00188-7. [DOI] [PubMed] [Google Scholar]
- Hogle JM, Curtin JJ. Sex differences in negative affective response during nicotine withdrawal. Psychophysiology. 2006;43:344–356. doi: 10.1111/j.1469-8986.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: similarities and dissimilarities. Addiction. 1994;89:1461–1470. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, McGeary J, Wooden A, Blumenthal T, Ito T. Startle magnitude and prepulse inhibition: effects of alcohol and attention. Psychopharmacology. 2003;167:235–241. doi: 10.1007/s00213-002-1332-7. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Niaura R, Swift R. The effects of smoking high nicotine cigarettes on prepulse inhibition, startle latency, and subjective responses. Psychopharmacology. 2000;150:244–252. doi: 10.1007/s002130000399. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biological Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jones GMM, Sahakian BJ, Levy R, Warburton DM, Gray JA. Effects of acute subcutaneous nicotine on attention, information processing and short-term memory in Alzeimers's disease. Psychopharmacology. 1992;108:437–442. doi: 10.1007/BF02247426. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Üstün TB. The World Mental Health (WMH) survey initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) Int J Methods Psychiatr Res. 2004;13:93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Checkley S, Gray J. Effect of cigarette smoking on prepulse inhibition of the acoustic startle reflex in healthy male smokers. Psychopharmacology. 1996;128:54–60. doi: 10.1007/s002130050109. [DOI] [PubMed] [Google Scholar]
- Lissek S, Dvir S, Baas JMP, Mc Dowell DJ, Pine DS, Shaywitz E, Grillon C. Pathological anxiety is associated with sustained anxiety to an unpredictably stressful context but not with phasic fear reactions to an explicit threat-cue 60th Annual Meeting of the Society of Biological Psychiatry; Atlanta, GA. 2005. [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–70. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Does cigarette smoking cause stress? The American psychologist. 1999;54:817–820. doi: 10.1037//0003-066x.54.10.817. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Garnham NJ. Comparative Mood States and Cognitive Skills of Cigarette Smokers, Deprived Smokers and Nonsmokers. Human Psychopharmacology. 1998;13:367–376. [Google Scholar]
- Pomerleau CS, Pomerleau OF. The effects of a psychological stressor on cigarette smoking and subsequent behavioral and physiological responses. Psychophysiology. 1987;24:278–285. doi: 10.1111/j.1469-8986.1987.tb00295.x. [DOI] [PubMed] [Google Scholar]
- Shiffman SS. Relapse following smoking cessation: a situational analysis. Journal of Consulting and Clinical Psychology. 1982;50:71–86. doi: 10.1037//0022-006x.50.1.71. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Auerbach P, Monroe SM, Hartston H, Geyer MA, Braff DL. Men are more inhibited than women by weak prepulses. Biological Psychiatry. 1993;34:253–260. doi: 10.1016/0006-3223(93)90079-s. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Carey G. Positive and negative affectivity and their relation to anxiety and depressive disorders. Journal of Abnormal Psychology. 1988;97:346–353. doi: 10.1037//0021-843x.97.3.346. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P. What happens to anxiety levels on giving up smoking? Am J Psychiatry. 1997;154:1589–92. doi: 10.1176/ajp.154.11.1589. [DOI] [PubMed] [Google Scholar]


